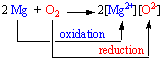
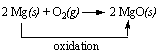what do we mean by oxidition and reduction in detail
Oxidation and reduction in terms of electron transfer:-
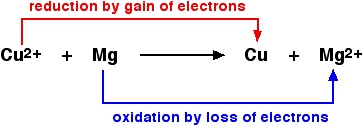
Definitions:-
- Oxidation is loss of electrons.
- Reduction is gain of electrons.
A simple example:-
The equation shows a simple redox reaction which can obviously be described in terms of oxygen transfer.
Copper(II) oxide and magnesium oxide are both ionic. The metals obviously aren 't. If you rewrite this as an ionic equation, it turns out that the oxide ions are spectator ions and you are left with: