what is meant by refining of metals? draw the diagram of electrolytic refining of copper and name the substances used as cathode ,anode and the electrolyte
Refining of Metals
Most metals obtained by the reduction process are not very pure. These have to be further refined or purified. Purification of the metal is the last step in metallurgy. Refining is based on the difference between the properties of metals and their impurities. The following processes are used for refining.
Liquation
In this method the metals are melted and made to go into the liquid state. Metals that have low melting points such as lead, tin etc., can be purified by this method. A sloping hearth of a furnace is used on which the metal is placed and melted. The temperature of the furnace is maintained slightly above the melting point of the metal. Due to the heat the pure metal melts and flows down, leaving behind infusible impurities having higher melting point.
Distillation
Oxidation
Electrorefining
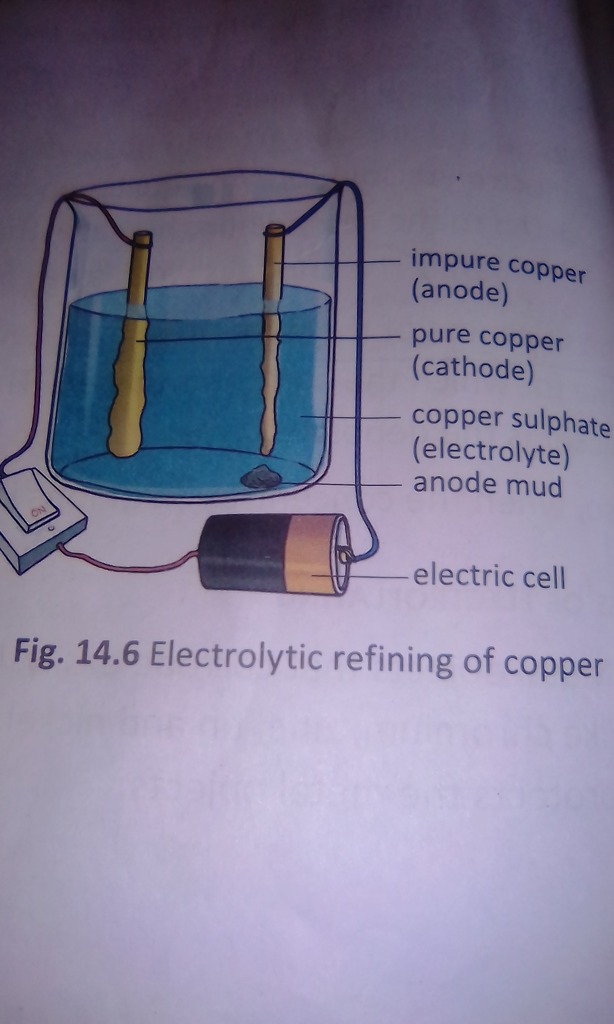
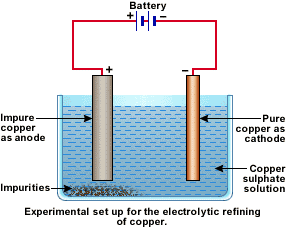
Electrolysis can be used for both extractions of metal (which cannot be separated by chemical reduction process) as well as for further purification of metals obtained by any other method. In the electrorefining process a block of impure metal is made the anode and a thin sheet of pure metal is made the cathode of of an electrolytic cell containing an aqueous solution of the metal salt. When electric current of a suitable voltage is passed, impure metal at the anode gets dissolved to deposit the pure metal at the cathode.
Metal ions from the anode enter the electrolyte as follows:
The impurities are left behind as anode mud near the anode. The anode finally disintegrates while the cathode gains in weight due to the collection of pure metal.
This method is used for refining volatile metals like copper, silver, tin, nickel that have boiling points lower than their impurities. e.g., zinc, mercury.
Remember
- An electrolyte is a compound (salt, acid or base), which in solution or in a molten state conducts an electric current and is simultaneously decomposed by it.
- Electrolytes are ionised into electrically charged ions, which carry the current.
- Charged ions move towards the oppositely charged electrodes to give up their electric charge and become atoms; these are either liberated or deposited at the electrodes.
ELECTROLYTIC REFINING OF COPPER:
- ANODE: Impure copper
- CATHODE: Pure copper
- ELECTROLYTE: Acidified copper sulphate solution.
Hope this helps u......
Thumbs up! if u r satisfied.........