what is normality?
NORMALITY IS THE CONCENTRATION TERM TO EXPRESS CONCENTRATION OF SOLUTE PRESENT IN THE SOLUTION.
NORMALITY IS DEFINED AS NO. OF GRAM EQUIVALENTS PER LITRE OF THE SOLUTION
MATHEMATICALLY WE MAY WRITE AS
NORMALITY=NO. OF GRAM EQUIVALENTS/VOLUME OF SOLUTION
FURTHUR
NO. OF GRM EQUIVALENTS=GIVEN MASS OF SOLUTE/EQUIVALENT MASS
FURTHER,
EQUIVALENT MASS=MOLECULAR MASS/Z-FACTOR



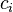 divided by an equivalence factor
divided by an equivalence factor  :
: