Be-Cl is polar but Becl2 is not why
BeCl2 is non polar due to its symmetric linear structure. Resultant dipole moment in symmetric molecules is always 0 and hence symmetric molecules are non polar in nature. In BeCl2, the dipole moment of one chlorine cancels out the dipole moment of other chlorine and hence the net dipole moment is 0. This is shown below:
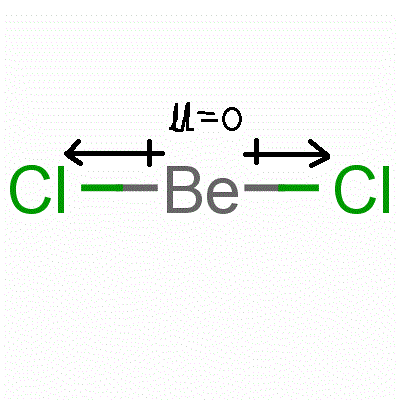
iOn the other hand, BeCl is also a linear molecule (if it exists) but the net dipole moment is non zero as only one bond is present and its dipole does not get cancelled.

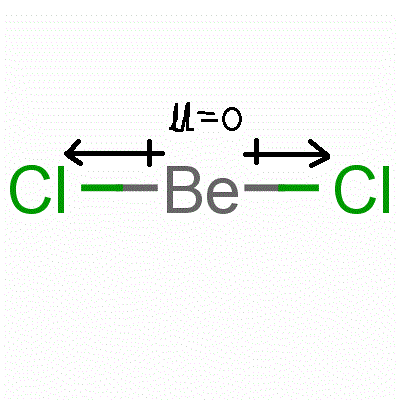
iOn the other hand, BeCl is also a linear molecule (if it exists) but the net dipole moment is non zero as only one bond is present and its dipole does not get cancelled.


