Explain chemical bonding in Potassium Ferrocyanide and Perchloric acid stepwise.
Dear Student,
The chemical bonding in Potassium Ferrocyanide and Perchloric acid can be explained as:
1. Potassium Ferrocyanide
The chemical structure of Potassium Ferrocyanide is as follows:

This compound contains covalent, coordinate and ionic bonds.
Nitrogen has three valence electrons, carbon has four valence electrons. So, because of minimal difference between nitrogen and carbon, they form three covalent bonds between them.
Now, iron has free valence orbitals. So, carbon donates a lone pair of electron to form a coordinate bond with the iron atom.Six carbon atoms form six coordinate bonds with a central iron atom. Since the carbon atom contains a free electron, the overall complex has a charge of -4.
So, this complex forms an ionic bond with four potassium atoms, which have a charge of +1 each.
Total covalent bonds are : 6 x 3 C-N = 18
Total coordinate bonds :6 C-Fe = 6
Total ionic bonds = 4
2. Perchloric acid :
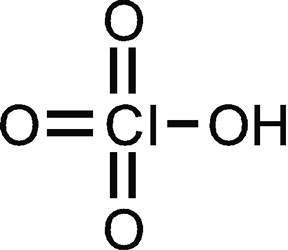
Oxygen requires two atoms to achieve octet configuration and chlorine requires one. Oxygen has two valence electrons and chlorine has three lone pairs and one valence electron. So, three oxygen atoms and the chlorine atoms share electrons to form two covalent bonds each and one oxygen atom forms one covalent bond with chlorine and one with hydrogen.
Thus, there are 3x2( Cl=O) + 1(Cl-O) and 1(O-H), that is, 8 covalent bonds in all.
Hope this simple explanation helps.
Let us know if you have further queries.
Regards
The chemical bonding in Potassium Ferrocyanide and Perchloric acid can be explained as:
1. Potassium Ferrocyanide
The chemical structure of Potassium Ferrocyanide is as follows:

This compound contains covalent, coordinate and ionic bonds.
Nitrogen has three valence electrons, carbon has four valence electrons. So, because of minimal difference between nitrogen and carbon, they form three covalent bonds between them.
Now, iron has free valence orbitals. So, carbon donates a lone pair of electron to form a coordinate bond with the iron atom.Six carbon atoms form six coordinate bonds with a central iron atom. Since the carbon atom contains a free electron, the overall complex has a charge of -4.
So, this complex forms an ionic bond with four potassium atoms, which have a charge of +1 each.
Total covalent bonds are : 6 x 3 C-N = 18
Total coordinate bonds :6 C-Fe = 6
Total ionic bonds = 4
2. Perchloric acid :

Oxygen requires two atoms to achieve octet configuration and chlorine requires one. Oxygen has two valence electrons and chlorine has three lone pairs and one valence electron. So, three oxygen atoms and the chlorine atoms share electrons to form two covalent bonds each and one oxygen atom forms one covalent bond with chlorine and one with hydrogen.
Thus, there are 3x2( Cl=O) + 1(Cl-O) and 1(O-H), that is, 8 covalent bonds in all.
Hope this simple explanation helps.
Let us know if you have further queries.
Regards

