Explain detailed process of fractional distillation
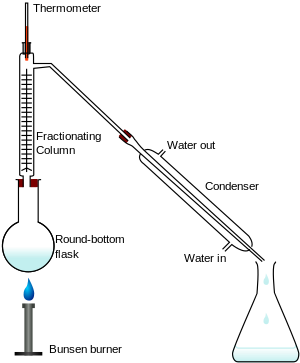
Fractional Distillation: It is a process of separation of a chemical mixture whose components have different boiling points.
It is an special type of distillation in which two miscible liquids having different boiling points but closed to each other are separated by fractionating column.
In this process, the the mixture is boiled in a round bottom flask, and the vapors of the boiling solution passes along the fractionating column. The temperature of the column is coolest at the top and gradually increases down as at the bottom it is attached with heating burner. Now, the components with higher boiling points condense on the column and hence after condensing they return to the solution. and the components with lower boiling points further pass through the column and are collected as they are more volatile.
This process is used for separating mixtures like : water and ethanol, or in production of gasoline from crude oil.