Explain the process Chromatography in detail for 5 marks
Dear Student,
Chromatography is a modern and sensitive techniques used for rapid and efficient separation or analysis of components of mixture and purification of compounds.
This technique was first used to separate coloured substances found in plant.
This is a physical method for separation.
Working Principle:
Separation of components of mixture is achieved by differential movement of individual components through a stationary phase under the influence of a mobile phase.
Based on this principle, Chromatography is classified as
(A) Adsorption Chromatography
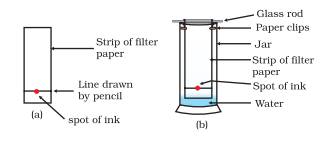
(B) Partition Chromatography (Paper Chromatography)
Adsorption Chromatography is further classified as
(i) Column Chromatography (ii) Thin layer Chromatography.
In this chromatography separation of components of a mixture is done over a thin layer of an adsorbent. A thin layer of adsorbent is spread over a glass sheet. The plate is called as Thin layer Chromatography plate. The solution containing different components is applied as a small spot at end of plate. Now the plate is kept in solvent. The solvent moves up along with the components of mixture. The components depending on their degree of adsorption moves up to different distance resulting in separation.
The relative adsorption of componenets represented in terms of retention factor i.e Rf value
Rf

Regards
Chromatography is a modern and sensitive techniques used for rapid and efficient separation or analysis of components of mixture and purification of compounds.
This technique was first used to separate coloured substances found in plant.
This is a physical method for separation.
Working Principle:
Separation of components of mixture is achieved by differential movement of individual components through a stationary phase under the influence of a mobile phase.
Based on this principle, Chromatography is classified as
(A) Adsorption Chromatography
(B) Partition Chromatography (Paper Chromatography)
Adsorption Chromatography is further classified as
(i) Column Chromatography (ii) Thin layer Chromatography.
In this chromatography separation of components of a mixture is done over a thin layer of an adsorbent. A thin layer of adsorbent is spread over a glass sheet. The plate is called as Thin layer Chromatography plate. The solution containing different components is applied as a small spot at end of plate. Now the plate is kept in solvent. The solvent moves up along with the components of mixture. The components depending on their degree of adsorption moves up to different distance resulting in separation.
The relative adsorption of componenets represented in terms of retention factor i.e Rf value
Rf

Regards