if E.W.G. or E.D.G.group is attached to -COOH W.R.T. resonance effect how it effects it's acidity e.g.Ph or NO2 etc.
Dear Student,
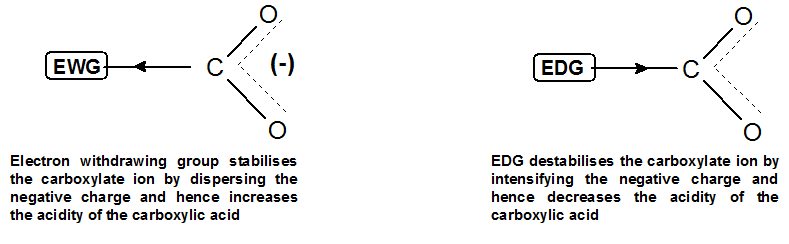
The acidity of the carboxylic acid can be modified by adding different groups on the alkyl portion of the acid.
Electron withdrawing groups make the molecule more acidic by stabilizing the negative charge on the conjugate base.
Whereas, electron donating groups destabilize the negative charge by donating electron density on the conjugate base, thus decreasing the acidity of the carboxylic acid.
Regards
The acidity of the carboxylic acid can be modified by adding different groups on the alkyl portion of the acid.
Electron withdrawing groups make the molecule more acidic by stabilizing the negative charge on the conjugate base.
Whereas, electron donating groups destabilize the negative charge by donating electron density on the conjugate base, thus decreasing the acidity of the carboxylic acid.

Regards

