In molecular orbital why the bonding orbital have lower energy than the antibonding orbital.....xplain

Dear student!
The molecular orbitals are formed due to linear combination of atomic orbitals where atomic orbitals are considered as an electron wave and the combination occurs as:
If ψAand ψBare the amplitudes of two atomic orbitals then, they combine by two ways:
1. Constructive interference: when both waves add together as, Φ = ψA+ ψB
2. Destructive interference: when two waves are subtracted as,Φ = ψA- ψB
Now, as their magnitudes are the square of amplitudes so,Φ 2 = (ψA+ ψB)2= ψA 2+ ψB 2+ 2ψAψB
While. second equation is ,Φ '2 = (ψA- ψB)2= ψA 2+ ψB 2- 2ψAψB
Φ 2 ψA 2+ ψB 2
Φ '2 A2+ ψB 2
Here, we see that, two new molecular orbitals formed where, constructive interference gives bonding MO and destructive interference gives antibonding MO.
The electron density is increased in bonding MO as the electrons are maximum found in bonded region while the probability is minimum in antibonding MO and so here the electron density is minimum.
So, antibonding MO has repulsion in atomic orbitals and so it requires much more energy to stabilize them and they are of minimum stability and higher energy.
But in case of bonding MO, the there is already attraction between the atomic orbitals and so they have lower energy and hence maximum stability.
Posted byShubhangini Kumari
Plz xplain as there is minimum electron density in antibonding MO then why , antibonding MO has repulsion in atomic orbitals also why there is attraction in bonding MO as electron density is maximum
Plz xplain!!
As we know that orbitals are represented by wave Functions. Molecular orbitals are formed by the linear combination of wave function of atomic orbital(LACO).Let me explain with example.
Each hydrogen atom in H2 has only the 1s orbital, so we add the two 1s wavefunctions. As you have learned in your study of atomic structure, atomic wavefunctions can have either plus or minus phases--this means the value of the wavefunction is either positive or negative. There are two ways to add the wavefunctions, either both in-phase (either both plus or both minus) or out-of-phase (one plus and the other minus). shows how atomic wavefunctions can be added together to produce molecular orbitals.
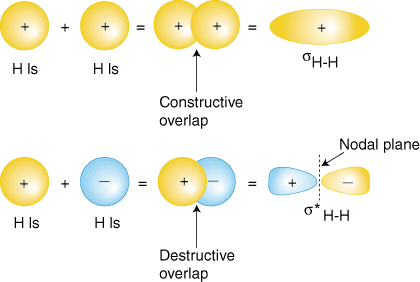
The in-phase overlap combination (top set of orbitals in ) produces a build-up of electron density between the two nuclei which results in a lower energy for that orbital. As it minimizes repulsion between the nucleus. The electrons occupying the δ H-H orbital represent the bonding pair of electrons and named a bonding molecular orbital. The other molecular orbital produced, δ * H-H shows a decrease in electron density between the nuclei reaching a value of zero at the midpoint between the nuclei where there is a nodal plane. Since their is minimum or no electron density in between the nuclei of atoms it cause repulsion of nuclei. δ * H-H orbital shows a decrease in bonding between the two nuclei, it is called an antibonding molecular orbital. Due to the decrease in electron density between the nuclei, the antibonding orbital is higher in energy than both the bonding orbital and the hydrogen 1s orbitals.

