Is ClF3 polar or non polar?
Dear Student,
Please find below the solution of your asked query:
ClF3 - Chlorine Trifluoride:
To find the polarity firstly we have to draw the Lewis dot structure:
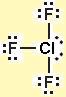
Keep asking!!
Regards
Please find below the solution of your asked query:
ClF3 - Chlorine Trifluoride:
To find the polarity firstly we have to draw the Lewis dot structure:
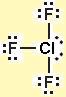
- Geometry of the compound Trigonal bipyramidal.
- Hybridization is sp3d.
- Now, as the molecular geometry of ClF3 is T-shaped with asymmetric charge distribution around the central atom.
- Therefore ClF3 is polar.
Keep asking!!
Regards

