Q= OUT OF THE FOLLOWING WHICH HAVE ZERO DIPOLE MOMENT AND WHY,,,,explain??
A) 1,1-dichloroethylene
B) cis-1,2-dichloroethylene
C) trans-1,2-dichloroethylene
D) none of these
Dear User
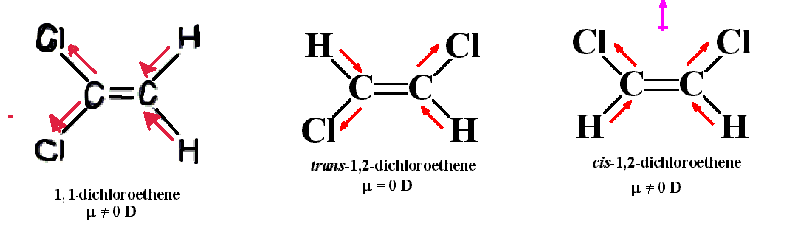
The correct answer is C) trans-1,2-dichloroethylene
Dipole moment is the vector sum of all the bond moments. In trans-1,2-dichloroethylene the bond moment of both Cl and both H points in opposite direction and thus the bond moments are cancelled. Therefore, the net dipole moment is 0.

The correct answer is C) trans-1,2-dichloroethylene
Dipole moment is the vector sum of all the bond moments. In trans-1,2-dichloroethylene the bond moment of both Cl and both H points in opposite direction and thus the bond moments are cancelled. Therefore, the net dipole moment is 0.

