ANODE RAYS:
· Travel in straight lines: They cast a shadow of the objects placed in their way.
· Produce mechanical effect: A paddle wheel placed in their path starts rotating.
· Rays are positively charged: Anode rays are deflected towards the negative plate of an electric field.
· The nature of the anode rays depends upon the gas taken in the discharge tube. Different gases give different types of positive rays, which contain particles having different masses and different charges. Therefore the (e/m) ratio is not constant for positive ray particles obtained from different gases.
CATHODE RAYS:
The big breakthrough came in the 1850's when Geissler invented a much better (mercury) pump. In 1869, Hittorf found that in a very good vacuum (0.01 mm Hg), the Faraday dark space expanded to fill the whole tube, and the cathode emitted rays that caused the glass to glow where they hit. In 1876, Goldstein called them "cathode rays". In 1879, an Englishman, William Crookes, declared that they must be particles of some sort, and demonstrated that they traveled in straight lines by inserting a Maltese cross in the tube, which cast a sharp shadow on the end of the tube, a demo still in common use 120 years later!
Were these cathode rays waves or particles? Hertz, with his student Lenard, discovered in 1891 that the rays could penetrate a thin aluminum plate, and detected them in the air just outside the tube. Hertz found experimentally that he could not deflect the rays with an electrostatic field applied from outside the tube, and they didn't affect a compass, so he concluded (wrongly) that they must be waves, not particles.
X RAYS:
All the activity described above did little to clarify the actual nature of x-rays. Roentgen himself found they were undeflected by a magnetic field, so were not charged particles like the cathode rays. On the other hand, they didn't exhibit any diffraction phenomena, so did not seem to be waves. Roentgen, and independently Thomson, found that the x-rays were ionizing radiation - as they passed through air, ions were created. A gold leaf electroscope exposed to the x-rays would lose its charge, as the newly created ions were attracted to the charged leaves.
In 1899, Haga and Wind noticed a slight broadening of an x-ray beam after it passed through a slit a few thousandths of a millimeter wide. This could be from diffraction if the wavelength were of order 10-10 meters. This problem was not resolved conclusively until 1912, when Laue made the observation that since the wavelength of x-rays was apparently similar to the distances between planes of atoms in a crystal, perhaps a crystal would act as a diffraction grating for x-rays. This turned out to be correct, and in fact is now the standard way of finding crystal structure.
It was concluded, then, that x-rays were "ultra- ultra violet light", as one French physicist had put it in 1896.
Once the usefulness of x-rays was established, techniques for producing them evolved rapidly. It was found that they were produced far more copiously if the cathode rays impinged on a piece of heavy metal, such as Molybdenum, rather than glass. The physical picture of x-ray production was that the electrons radiated as they suddenly decelerated on hitting the target, unloading their kinetic energy as radiation (plus some heating of the target). This deceleration radiation is called bremsstrahlung in German, and this word is sometimes used to describe it.
ALPHA PARTICLES:
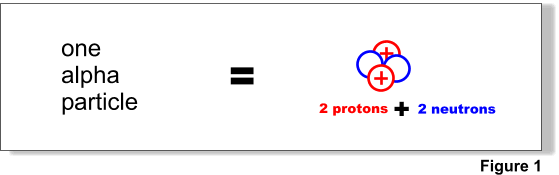
Alpha particles are composite particles consisting of two protons and two neutrons tightly bound together (Figure 1). They are emitted from the nucleus of some radionuclides during a form of radioactive decay, called alpha-decay. An alpha-particle is identical to the nucleus of a normal (atomic mass 4) helium atom or a doubly ionised helium atom.
What are the properties of alpha particles?
Alpha particles are relatively slow and heavy compared with other forms of nuclear radiation. The particles travels at 5 to 7 percent of the speed of light or 20,000,000 metres per second and has a mass approximately equivalent to 4 protons.


