what is construrction of nickel-cadmium cell?
The nickel–cadmium battery is a type of rechargeable battery. Active materials in nickel-cadmium cells are nickel hydrate (NiOOH) in the charged positive plate and sponge cadmium (Cd) in the charged negative plate. The electrolyte is
an aqueous potassium hydroxide (KOH) solution in concentration of 20-34 percent by weight pure KOH. The basic electrochemical reaction is :
At the cadmium electrode :

At the nickel electrode.
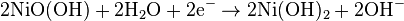
The net reaction during discharge is

During recharge, the reactions go from right to left
an aqueous potassium hydroxide (KOH) solution in concentration of 20-34 percent by weight pure KOH. The basic electrochemical reaction is :
At the cadmium electrode :

At the nickel electrode.
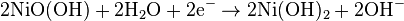
The net reaction during discharge is

During recharge, the reactions go from right to left

