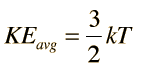what is law of equipartition of energy? please explain in simple words.
The theorem of equipartition of energy states that molecules in thermal equilibrium have the same average energy associated with each independent degree of freedom of their motion and that the energy is
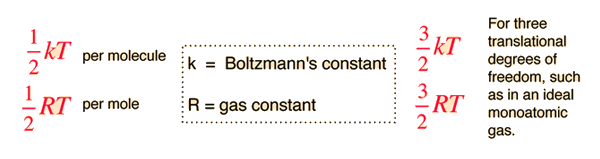
The equipartition result