https://www.meritnation.com/ask-answer/question/two-difference-between-metal-non-metal/materials-metals-and-non-metals/2667986
- 0
metal is a substance which donates electrons
and gains plus charge and contain mainy free electrons so is the good conductor of electricity
non metal accepts electrons and gains minus charge
and does not contain free electrons so is the bad conductor of electicity
hope so it helps u
so plz thumbs up
- 13
|
|
|
|
- 2
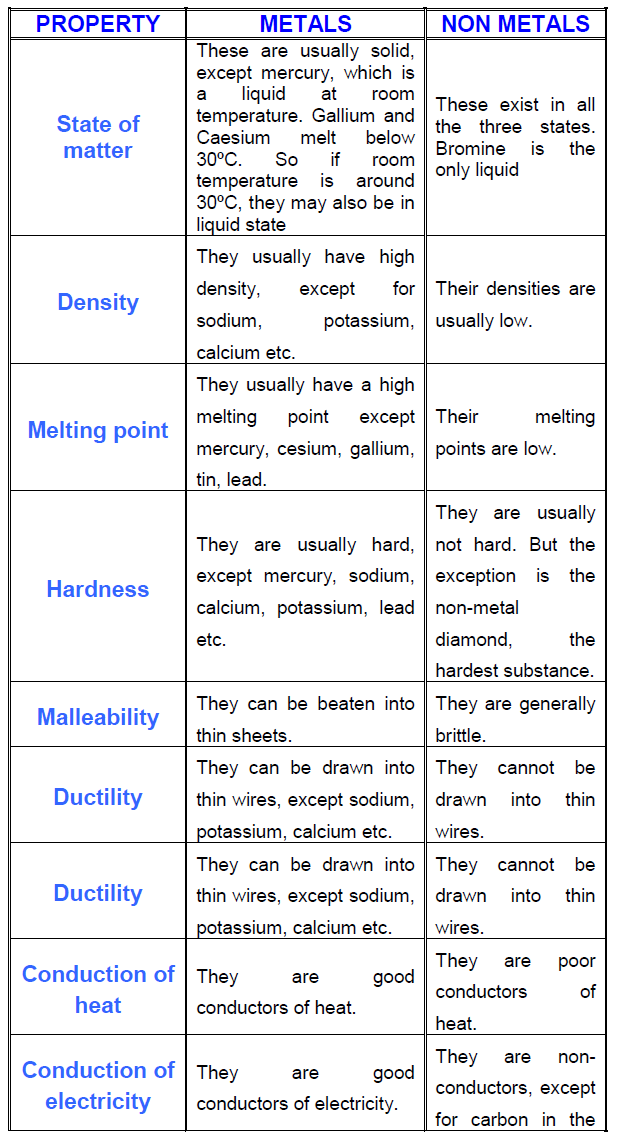
Elements can be classified as metals or non-metals on the basis of their properties.
Physical properties include:
- appearance
- density
- melting and boiling point
- conductivity of heat and electricity
- tensile strength (resistance to bending)
- malleability (ability to roll into sheets)
- ductility (ability to draw into a wire)
Chemical properties include:
- charge on ions formed from the element
- type of bonding found in the element's oxides and chlorides
- pH of the element's oxide
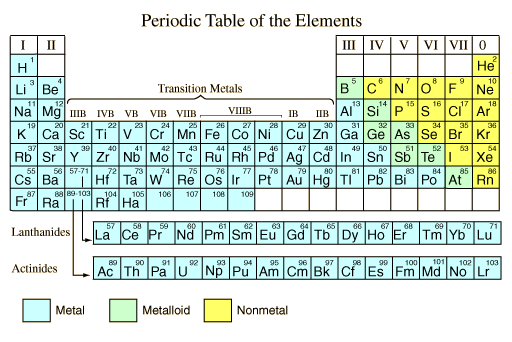
Metals are found on the left hand side of the Periodic Table while non-metals are found on the right hand side.
Properties of Metallic and Non-Metallic Elements
| Property | Metallic Elements | Non-Metallic Elements |
|---|---|---|
| Appearance (physical property) | lustrous | dull |
| Density (physical property) | moderate to high | low to moderate |
| Physical State (25oC, 101.3kPa) (physical property) | solid (except liquid mercury) | solid, liquid or gas |
| Melting and Boiling Point (physical property) | moderate to high | wide range |
| Heat and Electrical Conductivity (physical property) | good | poor (except graphite) |
| Tensile Strength (resistance to bending) (physical property) | high | brittle |
| Malleability (roll into sheets) (physical property) | malleable | not malleable |
| Ductility (draw into wire) (physical property) | ductile | not ductile |
| Charge on Ions (in general)* (chemical property) | forms positive ions | forms negative ions |
| Bonding in oxides and chlorides (chemical property) | usually ionic** | covalent |
| pH of oxides (chemical property) | usually basic*** | usually acidic |
*some non-metals can form positive or negative ions, eg, H+ and H-
**some metal oxides are covalent, eg, Al2O3
***some metal oxides are amphoteric (both acidic and basic), eg, Al2O3
Examples of Metals and Non-metals
| Property | Metal | Non-Metal | ||
|---|---|---|---|---|
| magnesium | zinc | oxygen | sulfur | |
| Density (g/mL) | 1.74 | 7.14 | 0.0013 | 2.07 |
| Melting Point (oC) | 650 | 419 | -219 | 113 |
| Electrical Conductivity (megaohm-1) | 23 | 16 | 0 | 10-21 |
| Charge on Ion | 2+ | 2+ | 2- | 2- |
| Bonding in Oxides | MgO ionic | ZnO ionic | O2 covalent | SO2 covalent |
Position of Metallic and Non-Metallic Elements in the Periodic Table
| Metals | Metals occur on the left hand side of the Periodic Table. |
| Non-metals | Non-metals occur on the right hand side of the Periodic Table. |
| Semi-metals (metalloids) | Semi-metals with properties in between metals and non-metals occur between these two groups. (B, Si, Ge, As, Sb, Te) |
| metals | non-metals | H | |||||||||||||||
| He | |||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Transition Metals | Ga | Ge | As | Se | Br | Kr | |||||||||
| Rb | Sr | Transition Metals | In | Sn | Sb | Te | I | Xe | |||||||||
| Cs | Ba | Transition Metals | Tl | Pb | Bi | Po | At | Rn | |||||||||
| Fr | Ra | Transition Metals | |||||||||||||||
- -2
Metals
Metals have many properties like they are lusterous and are hard. They are also very good conductors of heat and electricity. They are most of the time magnetic also. But this is not for every metal for example, Lithium is soft.
Examples of metals:
Cobalt, iron, copper, plutonium
Non-metals
Non metals also have many properties as well. Their properties are pretty much the opposite of metals. They are poor conductors.
Examples of non-metals:
Oxygen, hydrogen, neon
- 0


 .
.