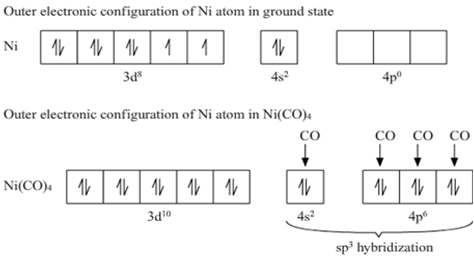
why Ni(Co)4 is outer complex!?
Dear Student
Regards
Outer orbital complex: When outer d-orbitals are used in hybridization.
As in the Ni(CO)4 Ni has zero oxidation state. So its inner d orbital is totally filled up by 10 electrons. Hence it requires outer d orbitals to make the bond.
Regards

