write the formation of sodium chloride and mg cholide
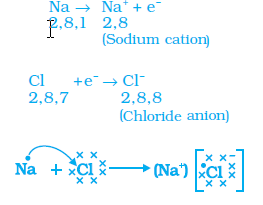
Sodium chloride can be formed as per the following equation
2Na + Cl2 -------> 2NaCl
The lewis dot structure is as follows
Magnesium chloride can be formed as follows
Mg + Cl2 ------> MgCl2
lewis dot structure for the same is



The formation of HCl can also be shown by one more equation
Mg + 2HCl ------> MgCl2 + H2
@ Arpita, very good attempt. Keep it up!!!!

