❖ In a chemical reaction, at least one of the following will occur:
❖ Chemical equation: A symbolic representation of the reactants, products and their physical states.
❖ Balanced chemical equation: Here, the total number of atoms on the reactant side is equal to the total number of atoms on the product side.
❖ How to balance an equation
❖ Exothermic reactions: In these types of reactions, heat is released.
❖ Endothermic reactions: In these types of reactions, heat is absorbed.
❖ Types of reactions
❖ Rancidity – The process of oxidation of fats and oils leading to the change of their taste and smell is called rancidity.Chapter 2: Acids, Bases and Salts
❖ Acids: These are the substances having sour taste. They turn the colour of blue litmus to red.
❖ Base: These are the substances having bitter taste. They turn the colour of red litmus to blue.
❖ Indicator: It is a dye that gives different colours in acids and/ or bases. Turmeric is a natural indicator.
❖ Reaction with metals:
Acid + Metals → Salt + Hydrogen gas
Zn + H2SO4 → ZnSO4 + H2
Base + Metals → Salt + Hydrogen gas
Zn + 2NaOH → Na2ZnO2 + H2
❖ Reaction of acids with metal carbonates and metal hydrogen carbonates
Metal carbonate/Metal hydrogen carbonate + Acid → Salt + Water + CO2
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
❖ Metal oxide + Acid
Metal oxide + Acid → Salt + Water
❖ Non-metal oxide + Base
Non-metal oxide + Base → Salt + Water
❖ Acid–Base reaction
Acid + Base → Salt + Water
NaOH + HCl → NaCl + H2O
❖ In water solution:
Acid releases H+ ion
H+ + H2O → H3O+
HCl + H2O → H3O+ + Cl–
Base releases OH– ion
pH 7 → Neutral solution
pH < 7 → Acidic solution
pH > 7 → Basic solution
Human body pH = 7.0 – 7.8
Change in pH in body causes → Tooth decay, stomach pain, burning pain (Honey bee)
❖ Common salt (NaCl) : Has pH = 7
.png)
❖ Bleaching powder → CaOCl2 (calcium oxychloride)
❖ Washing soda → Na2CO3.10H2O
❖ Plaster of Paris →
❖ Metals
❖ Non-metals
❖ Metals + Non-metals
.png)
❖ Physical properties of Ionic compounds
❖ Less active metals
❖ Moderately active metals
❖ Life processes: Continuously perform the functions of maintenance in living organisms.
Examples: digestion, respiration, circulation etc.
❖ Nutrition: Process of obtaining nutrients from the environment. Two types- autotrophic and heterotrophic
❖ Respiration
❖ Transportation
❖ Excretion: Involves removal of harmful metabolic wastes from the body.
❖ Control and coordination: Working together of various integrated body systems in response to changes in the body for maintenance of bodily functions.
❖ Parts of the nervous system
❖ Tropic movement
❖ Chemical coordination in plants
❖ Chemical coordination in animals
❖ Qualities of a good fuel/source of energy are:
❖ Factors to be considered for choosing fuel are:
❖ Conventional sources of energy:
❖ Non-conventional sources of energy
❖ Thermal power plant – Coal and petroleum are burned to produce heat
❖ Hydro power plant – (Renewable source)
❖ Technological improvement
❖ Properties of magnetic field lines
❖ Magnetic field lines of current carrying wire
❖ Right-hand thumb rule:
When thumb is in direction of current, the curl of fingers gives direction of circular magnetic field.
.png)
❖ Corkscrew rule
If one drives a corkscrew in the direction of the current, then the direction in which the handle is turned is the direction of the magnetic field on the magnetic field lines.
❖ Solenoid
Solenoid is a cylindrical coil having many turns of insulated wires wrapped closely. When current is passed through the coil, a magnetic field is produced along the axis of the coil.
.png)
❖ Direction of force on a current carrying conductor in a magnetic field can be given by Fleming’s left-hand rule.
.png)
❖ Application of magnetic force – Electric motor
When current is passed through a coil kept in a magnetic field, a force acts on it which rotates the electric motor.
❖ Electromagnetic Induction
Generation of a current in the conductor due to a varying magnetic field (moving magnet, or moving conductor)
❖ Direction of induced current in a conductor moving in a magnetic fierld can be given by Fleming’s right hand rule.
.png) Chapter 6: Electricity
Chapter 6: Electricity
❖ Electric current: Amount of charge flowing per unit time.
❖ Unit: I → Ampere
Q → Coulomb (C)
t = Second (s)
❖ Potential difference:
The potential difference between two separate points is defined as the work done to move a unit positive charge from one point to another.
❖ Unit:
❖ Ohm’s law:
Under constant physical conditions (i.e., constant temperature, pressure etc.), the current flowing through a conductor is directly proportional to the potential difference across the conductor.
❖ Net resistance of resistors in series connection
Rnet = R1 + R2 + R3 + … + Rn
❖ Net resistance of resistors in parallel
❖ Heating Effect of current, heat produced depends on:
❖ Application: Electric iron, toaster, fused wire
Fuse wire: a low-melting point wire connected in series with electric devices for safety.
❖ Electric power:
❖ Covalent bonds: The bonds formed by the sharing of electrons are known as covalent bonds.
❖ Carbon contains four electrons in its valence shell. It always forms covalent bonds as it is difficult for it to lose or gain four electrons in order to complete its octet.
❖ Allotropy: The property of an element to exist in different forms. Example: Diamond, graphite and buckminsterfullerene are the three allotropes of carbon.
.png)
❖ Catenation: The ability of an element to combine with itself through covalent bonds. Carbon can combine with itself to form chain, branched, and ring structures.
❖ Hydrocarbons: These are compounds of carbon and hydrogen.
❖ Saturated compounds: The compounds of carbon that contain only single bonds among carbon atoms. Example: alkanes
❖ Unsaturated compounds: The compounds of carbon having double and/ or triple bonds. Example: alkenes, alkynes
• The nomenclature of organic compounds is done by using a set of rules. Names of some common compounds are shown in the given table.
❖ Chemical properties of carbon compounds
❖ Ethanol (CH3CH2OH)
❖ Ethanoic acid (CH3COOH)
❖ The two ends of molecules of soaps and detergents are different. Their one end is hydrophilic and the other is hydrophobic. Presence of these two types of ends is responsible for the cleansing action of soaps.Chapter 2: Periodic Classification of Elements
❖ The earliest classification was based on grouping the known elements as metals and non-metals.
❖ Law of Triads: Given by Dobereiner. He was the first person to illustrate the relationship between the atomic masses of elements and their properties. A set of elements showing triads is as under:
.png)
❖ Law of Octaves: Given by Newlands. He arranged the known elements in the increasing order of their atomic masses. The law is applicable only to the elements having low atomic masses.
.png)
❖ Mendeleev’s periodic law: Mendeleev gave a periodic law which states that the properties of elements are a periodic function of their atomic masses.
❖ Modern periodic law: It states that the properties of elements are a periodic function of their atomic numbers, not their atomic masses..png)
❖ Heredity: Transmission of characteristics or traits from parents to offsprings
❖ Variations: Difference among individuals of a species and also, among offsprings of same parents. Variations are of two types- heritable and non-heritable
❖ Basis of heredity: each trait is influenced by both maternal and paternal DNA
❖ Mendel’s work
❖ Heredity at cellular level
❖ Sex determination in humans
❖ Evolution
❖ Evolutionary relationships
❖ Important components of the human eye
❖ Nearest focal distance of lens = 25 cm
❖ Common defects in eye
❖ Prism
.png)
❖ Dispersion
.png)
❖ Atmospheric refraction
❖ Scattering: Tyndall effect
Atmospheric particles, smoke, tiny water droplets, suspended particles of dust, and air molecules scatter sunlight. Therefore, the path of light becomes visible.
❖ Reproduction
❖ Asexual reproduction
.png)
.png)
.png)
.png)
.png)
❖ Sexual reproduction.png)
❖ Environment: natural surroundings and external conditions of an organism, which include all living and non-living factors that affect the organism
❖ Organism: is the basic unit of an ecological hierarchy, can be unicellular such as Amoeba and Paramecium or multicellular such as humans
❖ Population: a group of individuals of the same species inhabiting a given geographical area at a particular time and functioning as a unit
❖ Community: includes all individuals of different species living within a certain geographical area
❖ Ecosystem: includes both living and non-living components of an area
❖ Natural resources
❖ 3R principle to save environment
❖ Need to manage resources
❖ Type of Natural Resources
❖ Stakeholders in forest resources
❖ Water resources
❖ Coal and petroleum
❖ Sustainable Management
❖ Reflection of light is the change in the path of a light ray upon collision with an interface of two medium.
❖ Laws of reflection:
❖ Terms related to spherical mirrors
❖ Concave mirror and nature of image formed .png)
❖ Convex mirror and nature of image formed
❖ Mirror formula:
For concave, f → –ve, for convex, f → +ve
Magnification
❖ Laws of refraction
❖ Refractive index (R.I.)
❖ Terms related to spherical mirrors
❖ Convex lens and nature of image formed
❖ Concave lens and nature of image formed
❖ Lens Formula:
For concave lens f = –ve, convex lens f = +ve
❖ Magnification,
❖ Lens power P (Unit dipotre)
• Change in state
• Change in colour
• Evolution of a gas
• Change in temperature
• Change in colour
• Evolution of a gas
• Change in temperature
❖ Chemical equation: A symbolic representation of the reactants, products and their physical states.
❖ Balanced chemical equation: Here, the total number of atoms on the reactant side is equal to the total number of atoms on the product side.
❖ How to balance an equation
• Step - I: Write reactants and products
• Step – II: Balance the max. number of a particular atom on both sides
• Step – III: Balance other atoms
• Step – II: Balance the max. number of a particular atom on both sides
• Step – III: Balance other atoms
❖ Exothermic reactions: In these types of reactions, heat is released.
❖ Endothermic reactions: In these types of reactions, heat is absorbed.
❖ Types of reactions
• Combination reaction: Here, two or more reactants combine to form one single product.
Example:
• Decomposition reaction: Here, a single reactant breaks into several simple products.
Example:
• Displacement reaction: Here, one element replaces another element from a compound and forms a new compound.
Example:
• Double displacement reaction: The elements form two compounds which interchange their position.
Example:
• Oxidation and reduction reactions
Example:
• Decomposition reaction: Here, a single reactant breaks into several simple products.
Example:
• Displacement reaction: Here, one element replaces another element from a compound and forms a new compound.
Example:
• Double displacement reaction: The elements form two compounds which interchange their position.
Example:
• Oxidation and reduction reactions
⚬ Oxidation: In this type of reaction, a substance gains oxygen or releases hydrogen.
Example:
⚬ Reduction: In this type of reaction, a substance gains hydrogen or releases oxygen.
Example:
⚬ Redox reactions: Reactions where simultaneous oxidation and reduction reactions take place are called redox reactions. Example:
.png)
❖ Corrosion – The process of coating up of a metal by a layer of some other substance due to the presence of some external substances (such as acids and moisture) is called corrosion.Example:
⚬ Reduction: In this type of reaction, a substance gains hydrogen or releases oxygen.
Example:
⚬ Redox reactions: Reactions where simultaneous oxidation and reduction reactions take place are called redox reactions. Example:
.png)
❖ Rancidity – The process of oxidation of fats and oils leading to the change of their taste and smell is called rancidity.Chapter 2: Acids, Bases and Salts
❖ Acids: These are the substances having sour taste. They turn the colour of blue litmus to red.
❖ Base: These are the substances having bitter taste. They turn the colour of red litmus to blue.
❖ Indicator: It is a dye that gives different colours in acids and/ or bases. Turmeric is a natural indicator.
❖ Reaction with metals:
Acid + Metals → Salt + Hydrogen gas
Zn + H2SO4 → ZnSO4 + H2
Base + Metals → Salt + Hydrogen gas
Zn + 2NaOH → Na2ZnO2 + H2
❖ Reaction of acids with metal carbonates and metal hydrogen carbonates
Metal carbonate/Metal hydrogen carbonate + Acid → Salt + Water + CO2
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
❖ Metal oxide + Acid
Metal oxide + Acid → Salt + Water
❖ Non-metal oxide + Base
Non-metal oxide + Base → Salt + Water
❖ Acid–Base reaction
Acid + Base → Salt + Water
NaOH + HCl → NaCl + H2O
❖ In water solution:
Acid releases H+ ion
H+ + H2O → H3O+
HCl + H2O → H3O+ + Cl–
Base releases OH– ion
• Higher H+ concentration → Strong acid
• Lower H+ concentration → Weak acid
• Higher OH– concentration → Strong base
❖ pH → The measure of acidity or alkalinity (Measured on a scale of 0 to 14)• Lower H+ concentration → Weak acid
• Higher OH– concentration → Strong base
pH 7 → Neutral solution
pH < 7 → Acidic solution
pH > 7 → Basic solution
Human body pH = 7.0 – 7.8
Change in pH in body causes → Tooth decay, stomach pain, burning pain (Honey bee)
❖ Common salt (NaCl) : Has pH = 7
.png)
❖ Bleaching powder → CaOCl2 (calcium oxychloride)
• Preparation:
Ca(OH)2 + Cl2 → CaOCl2 + H2O
• Use:
Ca(OH)2 + Cl2 → CaOCl2 + H2O
• Use:
⚬
⚬ Oxidising agent
⚬ Disinfecting material
❖ Baking soda → NaHCO3 (Sodium hydrogen carbonate)
⚬ Oxidising agent
⚬ Disinfecting material
• Preparation:
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
• Use:
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
• Use:
⚬ Making baking powder
⚬ Ingredient for antacids
⚬ Soda–acid fire extinguisher
⚬ Ingredient for antacids
⚬ Soda–acid fire extinguisher
❖ Washing soda → Na2CO3.10H2O
• Preparation:
Na2CO3 + 10H2O → Na2CO3.H2O
• Use:
Na2CO3 + 10H2O → Na2CO3.H2O
• Use:
⚬ In glass, soap, paper industries
⚬ Making sodium compounds such as borax
⚬ As domestic cleaning agent
⚬ Removing permanent hardness of water
⚬ Making sodium compounds such as borax
⚬ As domestic cleaning agent
⚬ Removing permanent hardness of water
❖ Plaster of Paris →
• Preparation:
• Use:
• Use:
⚬ For making toys
⚬ For making decorations
⚬ For setting fractured bones
Chapter 3: Metals and Non-metals⚬ For making decorations
⚬ For setting fractured bones
❖ Metals
• Physical properties
⚬ Shining surface (in pure state) [called metallic lustre]
⚬ Generally hard [varies from metal to metal]
⚬ Malleability [ability to make thin sheets by beating]
⚬ Ductility [ability to make wire by drawing] [Gold is the most ductile element]
⚬ Good conductor of heat
⚬ High melting point
⚬ Conduct electricity
⚬ Sonorous [Produce sound]
⚬ Generally hard [varies from metal to metal]
⚬ Malleability [ability to make thin sheets by beating]
⚬ Ductility [ability to make wire by drawing] [Gold is the most ductile element]
⚬ Good conductor of heat
⚬ High melting point
⚬ Conduct electricity
⚬ Sonorous [Produce sound]
• Chemical properties
⚬ Combine with oxygen to form oxides: Example: 2Cu + O2 → 2CuO
Soluble metal oxides are called alkali. Na and K react very easily with O2. So, they are kept immersed in kerosene.
⚬ Reaction with water:
Metal + Water → Metal oxide + H2
If oxide is soluble, then metal hydroxide is formed.
2K + H2O → 2KOH + H2 + Heat
Soluble metal oxides are called alkali. Na and K react very easily with O2. So, they are kept immersed in kerosene.
⚬ Reaction with water:
Metal + Water → Metal oxide + H2
If oxide is soluble, then metal hydroxide is formed.
2K + H2O → 2KOH + H2 + Heat
⚬ Reaction with Acids
Metal + Dilute acid → Salt + H2
Reactivity: Mg > Al > Zn > Fe > Cu
Aqua regia: Freshly-prepared concentrated HCl+ and concentrated HNO3 in 3:1 ratio
It can dissolve gold and platinum.
⚬ Reaction with solutions of other metal salts: Displacement reactions
Reactivity series: K > Na > Ca > Mg > Al > Zn > Fe > Cu > An > Ag
Metal + Dilute acid → Salt + H2
Reactivity: Mg > Al > Zn > Fe > Cu
Aqua regia: Freshly-prepared concentrated HCl+ and concentrated HNO3 in 3:1 ratio
It can dissolve gold and platinum.
⚬ Reaction with solutions of other metal salts: Displacement reactions
Reactivity series: K > Na > Ca > Mg > Al > Zn > Fe > Cu > An > Ag
❖ Non-metals
• Physical properties
⚬ Do not have lustre
⚬ Generally, exist in liquid and gaseous states
⚬ Are neither malleable nor ductile
⚬ Bad conductors of heat and electricity
⚬ Are non-sonorous
⚬ Generally, exist in liquid and gaseous states
⚬ Are neither malleable nor ductile
⚬ Bad conductors of heat and electricity
⚬ Are non-sonorous
❖ Metals + Non-metals
.png)
❖ Physical properties of Ionic compounds
• They are usually found in solid state
• Hard [because of strong attraction force]
• Are usually brittle in nature
• High melting and boiling points
• Soluble in H2O; insoluble in kerosene, petrol
• Conduct electricity in H2O solution
❖ Extraction of metals
• Hard [because of strong attraction force]
• Are usually brittle in nature
• High melting and boiling points
• Soluble in H2O; insoluble in kerosene, petrol
• Conduct electricity in H2O solution
❖ Less active metals
❖ Moderately active metals
• Roasting – Heating of sulphide ore in excess air
2ZnS + 3O2 → 2ZnO + 2SO2
• Calcination – Heating of carbonate ores in limited air
ZnCO3 → ZnO + CO2
Chapter 4: Life Processes2ZnS + 3O2 → 2ZnO + 2SO2
• Calcination – Heating of carbonate ores in limited air
ZnCO3 → ZnO + CO2
❖ Life processes: Continuously perform the functions of maintenance in living organisms.
Examples: digestion, respiration, circulation etc.
❖ Nutrition: Process of obtaining nutrients from the environment. Two types- autotrophic and heterotrophic
• Autotrophic nutrition
⚬ Synthesis of food by photosynthesis
⚬ Photosynthesis equation:
⚬ Two phases of photosynthesis- light and dark reactions
⚬ Light reaction: light energy absorbed, H2O split into H2 and O2, ATP and NADPH2 synthesized
⚬ Dark reaction: CO2 reduced to carbohydrates
⚬ Photosynthesis equation:
⚬ Two phases of photosynthesis- light and dark reactions
⚬ Light reaction: light energy absorbed, H2O split into H2 and O2, ATP and NADPH2 synthesized
⚬ Dark reaction: CO2 reduced to carbohydrates
• Heterotrophic nutrition
⚬ Generally derive energy from plants and animal sources
⚬ Mainly of three types: holozoic, parasitic, and saprophytic
⚬ Digestion: mechanical and chemical reduction of ingested nutrients
⚬ Human digestive system: consists of the long alimentary canal
⚬ Parts of alimentary canal
.png)
⚬ Mainly of three types: holozoic, parasitic, and saprophytic
⚬ Digestion: mechanical and chemical reduction of ingested nutrients
⚬ Human digestive system: consists of the long alimentary canal
⚬ Parts of alimentary canal
.png)
⚬ Accessory organs: pancreas, liver
❖ Respiration
• Enzymatically-controlled energy released from the breakdown of organic substances
• Two types- aerobic and anaerobic
• Aerobic respiration
• Two types- aerobic and anaerobic
• Aerobic respiration
⚬ Oxidation of food materials with the help of oxygen
⚬ Yields 36 ATP
⚬ First step- glycolysis (occurs in the cytoplasm), 2 pyruvate molecules produced
⚬ Second step- acetyl CoA produced
⚬ Third step- Kreb’s cycle inside the mitochondrial matrix, energy produced
⚬ Last step- energy converted to ATP by ATP synthase enzyme
⚬ Yields 36 ATP
⚬ First step- glycolysis (occurs in the cytoplasm), 2 pyruvate molecules produced
⚬ Second step- acetyl CoA produced
⚬ Third step- Kreb’s cycle inside the mitochondrial matrix, energy produced
⚬ Last step- energy converted to ATP by ATP synthase enzyme
• Anaerobic respiration
⚬ Oxidation of nutrients without utilizing molecular oxygen
⚬ Yields 2 ATP
⚬ First step- glycolysis (occurs in the cytoplasm), 2 pyruvate produced
⚬ Second step- break down of pyruvic acid into waste products
⚬ Yields 2 ATP
⚬ First step- glycolysis (occurs in the cytoplasm), 2 pyruvate produced
⚬ Second step- break down of pyruvic acid into waste products
• Human respiration
.png)
.png)
⚬ Bronchioles divide to form many alveoli
⚬ Alveoli are sites of gas exchange
⚬ O2 present in alveolar blood vessels transported to body cells
⚬ Alveoli are sites of gas exchange
⚬ O2 present in alveolar blood vessels transported to body cells
❖ Transportation
• A liquid medium is required
• Transportation in humans
• Transportation in humans
⚬ Blood, lymph- involved in transportation
⚬ Components of blood- RBCs, WBCs, platelets, and plasma
⚬ Two types of blood vessels- arteries and veins
⚬ Arteries carry oxygenated blood, except pulmonary artery
⚬ Veins carry deoxygenated blood, except pulmonary vein
⚬ Human heart divided into four chambers – right auricle, right ventricle, left auricle, and left ventricle
.png)
⚬ Components of blood- RBCs, WBCs, platelets, and plasma
⚬ Two types of blood vessels- arteries and veins
⚬ Arteries carry oxygenated blood, except pulmonary artery
⚬ Veins carry deoxygenated blood, except pulmonary vein
⚬ Human heart divided into four chambers – right auricle, right ventricle, left auricle, and left ventricle
.png)
⚬ Right side of the heart receives deoxygenated blood
⚬ Left side of the heart receives oxygenated blood
⚬ Left side of the heart receives oxygenated blood
• Transportation in plants
⚬ Transport of water-xylem
⚬ Transport of food- phloem
⚬ Transport of food- phloem
❖ Excretion: Involves removal of harmful metabolic wastes from the body.
• Excretion in humans
.png)
.png)
⚬ Nitrogenous wastes such as urea and uric acid are removed
⚬ Nephron- basic filtration unit
⚬ Main components of the nephron are: glomerulus, Bowman’s capsule, renal tube
.png)
Chapter 5: Control and Coordination⚬ Nephron- basic filtration unit
⚬ Main components of the nephron are: glomerulus, Bowman’s capsule, renal tube
.png)
❖ Control and coordination: Working together of various integrated body systems in response to changes in the body for maintenance of bodily functions.
• Nervous and muscular tissues provide control and coordination
• Neurons -functional units of the nervous system, conduct messages in the form of impulses
• Synapse- a small gap between the axon of one neuron and the dendrite of the next neuron
• Three types of responses of the nervous system
• Reflex action
• Neurons -functional units of the nervous system, conduct messages in the form of impulses
• Synapse- a small gap between the axon of one neuron and the dendrite of the next neuron
• Three types of responses of the nervous system
• Reflex action
⚬ Automated action in response to a stimulus
⚬ Possible due to quick detection by sensory receptors and the resultant movement of muscles
⚬ Reflex arc situated in the spinal cord
⚬ Possible due to quick detection by sensory receptors and the resultant movement of muscles
⚬ Reflex arc situated in the spinal cord
• Voluntary action: Actions such as writing, talking etc. that are under the control of the body.
• Involuntary action: Actions such as breathing, digestion etc. that are not under conscious control
• Involuntary action: Actions such as breathing, digestion etc. that are not under conscious control
❖ Parts of the nervous system
• Human nervous system divided into- central nervous system (CNS) and peripheral nervous system (PNS)
• CNS consists of the brain and spinal cord
• PNS consists of the nerves that connects the CNS to different parts of the body
• The Brain, spinal cord, and nerves are the important parts of the nervous system
• CNS consists of the brain and spinal cord
• PNS consists of the nerves that connects the CNS to different parts of the body
• The Brain, spinal cord, and nerves are the important parts of the nervous system
• Brain
.png)
.png)
⚬ Human brain is classified into- forebrain, midbrain, and hindbrain
⚬ Forebrain- cerebrum, thalamus, and hypothalamus
⚬ Midbrain
⚬ Hindbrain- pons, medulla, and cerebellum
⚬ Forebrain- cerebrum, thalamus, and hypothalamus
⚬ Midbrain
⚬ Hindbrain- pons, medulla, and cerebellum
❖ Tropic movement
• Directional movement of a specific part of the plant in response to an external stimulus
• Phototropism- response to light
• Geotropism- response to gravity
• Hydrotropism- response to water
• Chemotropism- response to chemicals
• Thigmotropism- response to touch
• Phototropism- response to light
• Geotropism- response to gravity
• Hydrotropism- response to water
• Chemotropism- response to chemicals
• Thigmotropism- response to touch
❖ Chemical coordination in plants
• Growth and development in plants is possible because of growth hormones or phytohormones
• Auxin, Gibberellin, cytokinin, abscisic acid and ethylene are examples of phytohormones
• Auxin, Gibberellin, cytokinin, abscisic acid and ethylene are examples of phytohormones
❖ Chemical coordination in animals
• Carried out with the help of hormones
• Hormones are secreted by endocrine glands such as the pituitary gland, thyroid gland, adrenal gland, pancreas etc.
Chapter 8: Sources of Energy• Hormones are secreted by endocrine glands such as the pituitary gland, thyroid gland, adrenal gland, pancreas etc.
❖ Qualities of a good fuel/source of energy are:
• That would do a large amount of work per unit volume or mass
• Easily accessible
• Easy to store and transport
• Economical
• Easily accessible
• Easy to store and transport
• Economical
❖ Factors to be considered for choosing fuel are:
• How much heat it produces
• Less smoke generation
• Easy availability
• Less smoke generation
• Easy availability
❖ Conventional sources of energy:
• Fossil fuels – Coal, petroleum and natural gas
• Advantages
• Advantages
⚬ Easy availability
⚬ Generate heat that is easily converted into electricity
⚬ Generate heat that is easily converted into electricity
• Disadvantages
⚬ Non-renewable
⚬ Limited reserve
⚬ Cause air pollution
⚬ Limited reserve
⚬ Cause air pollution
❖ Non-conventional sources of energy
• Solar energy – Solar cooker, solar water heater (very efficient for small scale electricity production)
• Tidal energy, wave energy, ocean thermal energy
• Geothermal energy – Heat energy inside the earth
• Wind energy
• Nuclear energy – Not dependent on solar energy, never-ending source, very efficient source, more environment friendly
• Tidal energy, wave energy, ocean thermal energy
• Geothermal energy – Heat energy inside the earth
• Wind energy
• Nuclear energy – Not dependent on solar energy, never-ending source, very efficient source, more environment friendly
❖ Thermal power plant – Coal and petroleum are burned to produce heat
❖ Hydro power plant – (Renewable source)
• Problems – Limited places for construction (only Hilly areas)
❖ Technological improvement
• Bio-mass – Charcoal, cow-dung, vegetable waste, sewage
• Wind energy – Environment friendly, renewable
Chapter 7: Magnetic Effects of Current• Wind energy – Environment friendly, renewable
❖ Properties of magnetic field lines
• Originate from the North pole and end at the South pole [outside the magnet]
• They are closed continuous lines
• Density of the lines increases near the poles and decreases away from the poles
• Lines never cross each other
• They are closed continuous lines
• Density of the lines increases near the poles and decreases away from the poles
• Lines never cross each other
❖ Magnetic field lines of current carrying wire
• It is circular with axis as the wire.
• Varies with distance from wire. (Inversely proportional)
• Direction depends on direction of current.
• Deflection of compass near a conductor (Shown by arrow):
.png)
• Varies with distance from wire. (Inversely proportional)
• Direction depends on direction of current.
• Deflection of compass near a conductor (Shown by arrow):
.png)
❖ Right-hand thumb rule:
When thumb is in direction of current, the curl of fingers gives direction of circular magnetic field.
.png)
❖ Corkscrew rule
If one drives a corkscrew in the direction of the current, then the direction in which the handle is turned is the direction of the magnetic field on the magnetic field lines.
❖ Solenoid
Solenoid is a cylindrical coil having many turns of insulated wires wrapped closely. When current is passed through the coil, a magnetic field is produced along the axis of the coil.
.png)
❖ Direction of force on a current carrying conductor in a magnetic field can be given by Fleming’s left-hand rule.
.png)
❖ Application of magnetic force – Electric motor
When current is passed through a coil kept in a magnetic field, a force acts on it which rotates the electric motor.
❖ Electromagnetic Induction
Generation of a current in the conductor due to a varying magnetic field (moving magnet, or moving conductor)
• Application – AC/DC generator
❖ Direction of induced current in a conductor moving in a magnetic fierld can be given by Fleming’s right hand rule.
.png) Chapter 6: Electricity
Chapter 6: Electricity❖ Electric current: Amount of charge flowing per unit time.
❖ Unit: I → Ampere
Q → Coulomb (C)
t = Second (s)
❖ Potential difference:
The potential difference between two separate points is defined as the work done to move a unit positive charge from one point to another.
❖ Unit:
❖ Ohm’s law:
Under constant physical conditions (i.e., constant temperature, pressure etc.), the current flowing through a conductor is directly proportional to the potential difference across the conductor.
• Current ∝ potential difference (V ∝ I)
V = IR Where, R = resistance
• Unit of resistance (R) →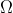 (Ohm)
(Ohm)
❖ Factors on which resistance depends
V = IR Where, R = resistance
• Unit of resistance (R) →
• R ∝ l, when area of cross-section and material are constant l = length
• R ∝ A, when l and material are constant A = perpendicular cross-section
• Overall,
• Or, , where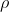 is resistivity which is different for different material
is resistivity which is different for different material
• Resistivity of a substance is equal to the resistance of a unit square of that substance.
• Unit (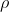 ) →
) → 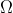 . m
. m
• R ∝ A, when l and material are constant A = perpendicular cross-section
• Overall,
• Or, , where
• Resistivity of a substance is equal to the resistance of a unit square of that substance.
• Unit (
❖ Net resistance of resistors in series connection
Rnet = R1 + R2 + R3 + … + Rn
❖ Net resistance of resistors in parallel
❖ Heating Effect of current, heat produced depends on:
• Potential difference (V)
• Electric current (I)
• Time for which current passes (t)
• Electric energy = VIt
• It can be written as: E = I2Rt
• Unit – 1 kWh = 3.6 × 106 J
• Electric current (I)
• Time for which current passes (t)
• Electric energy = VIt
• It can be written as: E = I2Rt
• Unit – 1 kWh = 3.6 × 106 J
❖ Application: Electric iron, toaster, fused wire
Fuse wire: a low-melting point wire connected in series with electric devices for safety.
❖ Electric power:
• Unit: 1 W = 1V × 1A
Chapter 1: Carbon and Its Compounds❖ Covalent bonds: The bonds formed by the sharing of electrons are known as covalent bonds.
❖ Carbon contains four electrons in its valence shell. It always forms covalent bonds as it is difficult for it to lose or gain four electrons in order to complete its octet.
❖ Allotropy: The property of an element to exist in different forms. Example: Diamond, graphite and buckminsterfullerene are the three allotropes of carbon.
.png)
❖ Catenation: The ability of an element to combine with itself through covalent bonds. Carbon can combine with itself to form chain, branched, and ring structures.
❖ Hydrocarbons: These are compounds of carbon and hydrogen.
❖ Saturated compounds: The compounds of carbon that contain only single bonds among carbon atoms. Example: alkanes
❖ Unsaturated compounds: The compounds of carbon having double and/ or triple bonds. Example: alkenes, alkynes
• Homologous series: A series of carbon compounds having different numbers of carbon atoms, but containing the same functional group. Some functional groups in carbon compounds are shown in the given table.
| Hetero atom | Name of functional group | Formula of functional group |
| Chlorine/Bromine | Halo- (Chloro/Bromo) | –Cl, –Br |
| Oxygen | Alcohol | –OH |
| Aldehyde | –CHO | |
| Ketone | >C=O | |
| Carboxylic acid | –COOH |
• The nomenclature of organic compounds is done by using a set of rules. Names of some common compounds are shown in the given table.
| Functional group | Prefix/Suffix | Example | |
| 1. | Halogen | Prefix: chloro, bromo, etc. |  |
 |
|||
| 2. | Alcohol | Suffix: -ol |  |
| 3. | Aldehyde | Suffix: -al |  |
| 4. | Ketone | Suffix: -one |  |
| 5. | Carboxylic acid | Suffix: -oic acid |  |
| 6. | Double bond (alkenes) | Suffix: -ene |  |
| 7. | Triple bond (alkynes) | Suffix: -yne |  |
❖ Chemical properties of carbon compounds
• Combustion reaction:
Carbon burns in air to form carbon dioxide and hydrocarbons burn in air to give carbon dioxide and water. Heat and light are also released in these processes.
CH4 + O2 → CO2 + H2O + Heat and light
Carbon burns in air to form carbon dioxide and hydrocarbons burn in air to give carbon dioxide and water. Heat and light are also released in these processes.
CH4 + O2 → CO2 + H2O + Heat and light
• Oxidation reaction:
Combustion of carbon to form carbon dioxide is an oxidation reaction. When alcohols are oxidised, carboxylic acids are obtained.
Combustion of carbon to form carbon dioxide is an oxidation reaction. When alcohols are oxidised, carboxylic acids are obtained.
⚬ Addition reaction:
Unsaturated hydrocarbons yield saturated hydrocarbons when reacted with hydrogen in the presence of catalysts.
⚬ Substitution reaction:
Under specific conditions, hydrogen atoms present in hydrocarbons can be replaced by atoms of other elements like chlorine and bromine.
Unsaturated hydrocarbons yield saturated hydrocarbons when reacted with hydrogen in the presence of catalysts.
⚬ Substitution reaction:
Under specific conditions, hydrogen atoms present in hydrocarbons can be replaced by atoms of other elements like chlorine and bromine.
❖ Ethanol (CH3CH2OH)
• Physical properties
⚬ Liquid at room temperature
⚬ Is a good solvent
⚬ Soluble in water in all proportions
⚬ Is a good solvent
⚬ Soluble in water in all proportions
• Chemical properties
⚬ Reacts with sodium metal to release hydrogen gas
⚬ Reacts with conc. H2SO4 to form ethene
⚬ Reacts with conc. H2SO4 to form ethene
❖ Ethanoic acid (CH3COOH)
• Physical properties
⚬ Has a melting point of 290 K
⚬ 5-8% solution of acetic acid is known as vinegar
⚬ Is a weak acid
⚬ 5-8% solution of acetic acid is known as vinegar
⚬ Is a weak acid
• Chemical properties
⚬ Esterification reaction
The reaction reverses itself in the presence of a base and is called saponificaiton reaction.
The reaction reverses itself in the presence of a base and is called saponificaiton reaction.
❖ The two ends of molecules of soaps and detergents are different. Their one end is hydrophilic and the other is hydrophobic. Presence of these two types of ends is responsible for the cleansing action of soaps.Chapter 2: Periodic Classification of Elements
❖ The earliest classification was based on grouping the known elements as metals and non-metals.
❖ Law of Triads: Given by Dobereiner. He was the first person to illustrate the relationship between the atomic masses of elements and their properties. A set of elements showing triads is as under:
.png)
❖ Law of Octaves: Given by Newlands. He arranged the known elements in the increasing order of their atomic masses. The law is applicable only to the elements having low atomic masses.
.png)
❖ Mendeleev’s periodic law: Mendeleev gave a periodic law which states that the properties of elements are a periodic function of their atomic masses.
• Achievements of Mendeleev’s periodic table:
⚬ Mendeleev left some gaps in his periodic table so that the undiscovered elements could get a place in it without disturbing the positions of the other elements.
⚬ Noble metals were not discovered at that time. When they were discovered later, they got a place in Mendeleev’s table without disturbing the positions of the other elements.
⚬ Noble metals were not discovered at that time. When they were discovered later, they got a place in Mendeleev’s table without disturbing the positions of the other elements.
• Limitations of Mendeleev’s periodic table:
⚬ It failed to explain the position of hydrogen.
⚬ It was not able to explain the position of isotopes.
⚬ In the table some elements having higher mass were kept before the elements having lesser atomic mass.
⚬ It was not able to explain the position of isotopes.
⚬ In the table some elements having higher mass were kept before the elements having lesser atomic mass.
❖ Modern periodic law: It states that the properties of elements are a periodic function of their atomic numbers, not their atomic masses.
• The modern periodic table consists of 7 periods and 18 groups.
• Elements having the same valence shell are present in the same period. Elements having the same number of valence electrons are present in the same group.
• The Metals are present on the right-hand side of the periodic table, whereas non-metals are present on the left-hand side of the periodic table.
• Elements having the same valence shell are present in the same period. Elements having the same number of valence electrons are present in the same group.
• The Metals are present on the right-hand side of the periodic table, whereas non-metals are present on the left-hand side of the periodic table.
.png)
• The atomic size as well as metallic character of elements increases on moving down the group and decreases on moving from left to right in a period.
Chapter 4: Heredity and Evolution❖ Heredity: Transmission of characteristics or traits from parents to offsprings
❖ Variations: Difference among individuals of a species and also, among offsprings of same parents. Variations are of two types- heritable and non-heritable
❖ Basis of heredity: each trait is influenced by both maternal and paternal DNA
❖ Mendel’s work
• Proposed that heredity controlled by genes
• Performed experiments on garden peas (Pisum sativum)
• Used seven contrasting pairs of characters or traits
• Dominant trait: able to express itself over another contrasting trait
• Recessive trait: unable to express its effect in the presence of a dominant trait
• Mendel represented- dominant trait as upper case (e.g.,T for tallness) and recessive trait as lower case (e.g., t for shortness)
• Homozygous: when the factors or genes of a trait are similar e.g.,TT or tt
• Heterozygous: when the factors or genes of a trait are different e.g., Tt
• Genotype: genetic constitution of an organism e.g., pure tall- TT
• Phenotype: observable traits or characteristics of an organism e.g., tallness, shortness etc.
• Genotypic ratio: expected ratio of genotypes produced by a particular cross
• Phenotypic ratio: expected ratio of phenotypes produced by a particular cross
• Monohybrid cross: involves only one pair of contrasting characters
• Dihybrid cross: involves two pairs of contrasting characters
• Stages of Mendel’s experiment
• Performed experiments on garden peas (Pisum sativum)
• Used seven contrasting pairs of characters or traits
• Dominant trait: able to express itself over another contrasting trait
• Recessive trait: unable to express its effect in the presence of a dominant trait
• Mendel represented- dominant trait as upper case (e.g.,T for tallness) and recessive trait as lower case (e.g., t for shortness)
• Homozygous: when the factors or genes of a trait are similar e.g.,TT or tt
• Heterozygous: when the factors or genes of a trait are different e.g., Tt
• Genotype: genetic constitution of an organism e.g., pure tall- TT
• Phenotype: observable traits or characteristics of an organism e.g., tallness, shortness etc.
• Genotypic ratio: expected ratio of genotypes produced by a particular cross
• Phenotypic ratio: expected ratio of phenotypes produced by a particular cross
• Monohybrid cross: involves only one pair of contrasting characters
• Dihybrid cross: involves two pairs of contrasting characters
• Stages of Mendel’s experiment
⚬ Selection of parents- true breeding with contrasting pairs of traits e.g., pure tall (TT) and pure dwarf (tt) pea plants were selected
⚬ Obtaining F1 plants- F1 generation is the first filial generation, formed after crossing desirable parents e.g., crossing pure tall (TT) and dwarf (tt) plants gives heterozygous tall (Tt) F1 plants
⚬ Obtaining F1 plants- F1 generation is the first filial generation, formed after crossing desirable parents e.g., crossing pure tall (TT) and dwarf (tt) plants gives heterozygous tall (Tt) F1 plants
⚬ Self-pollination of F1 plants- involves crossing F1 plants to obtain F2 plants
• Conclusions of Mendel’s experiment
⚬ Each characteristic in an organism is represented by two factors
⚬ Two factors are- dominant and recessive
⚬ Two contrasting factors when present in an individual do not blend
⚬ When more than two factors are involved, they are independently inherited
⚬ Two factors are- dominant and recessive
⚬ Two contrasting factors when present in an individual do not blend
⚬ When more than two factors are involved, they are independently inherited
❖ Heredity at cellular level
• DNA associates with proteins to form chromosomes
• Every somatic (body) cell of the human body has 23 pairs (46) of chromosomes
• Autosomes- first 22 pairs of chromosomes that do not determine the sex of an individual
• Sex chromosomes- last pair of chromosomes, represented as X and Y
• Females have two X chromosomes, XX
• Males have one X and one Y chromosome, XY
• Every somatic (body) cell of the human body has 23 pairs (46) of chromosomes
• Autosomes- first 22 pairs of chromosomes that do not determine the sex of an individual
• Sex chromosomes- last pair of chromosomes, represented as X and Y
• Females have two X chromosomes, XX
• Males have one X and one Y chromosome, XY
❖ Sex determination in humans
• Gametes receive half of the chromosomes
• Male gametes have 22 autosomes and either X or Y sex chromosome
• Male gametes can be of two types, 22 + X or 22 + Y
• Female gametes can be of only one type, 22 + X
• Sex of a baby is determined by the type of the male gamete (X or Y) that fuses with the female gamete
• Male gametes have 22 autosomes and either X or Y sex chromosome
• Male gametes can be of two types, 22 + X or 22 + Y
• Female gametes can be of only one type, 22 + X
• Sex of a baby is determined by the type of the male gamete (X or Y) that fuses with the female gamete
❖ Evolution
• Changes in inherited traits from one generation to the next in a species
• Variations leads to evolution
• Speciation- formation of new species
• Causes of evolution
• Variations leads to evolution
• Speciation- formation of new species
• Causes of evolution
⚬ Natural selection: a process that results in an increased survival and reproductive success of individuals that are well adjusted to the environment
⚬ Genetic drift: accidental change in the frequency of genes in a small population
⚬ Acquired traits: a trait that an individual experiences during his lifetime a) involves changes in non-reproductive tissues b) cannot be passed on to the progeny
⚬ Inherited traits: distinguishing qualities or characteristics that one acquires from ancestors (i) involves changes in DNA (ii) transmitted to progeny
⚬ Genetic drift: accidental change in the frequency of genes in a small population
⚬ Acquired traits: a trait that an individual experiences during his lifetime a) involves changes in non-reproductive tissues b) cannot be passed on to the progeny
⚬ Inherited traits: distinguishing qualities or characteristics that one acquires from ancestors (i) involves changes in DNA (ii) transmitted to progeny
❖ Evolutionary relationships
• Homologous organs: similar in origin, but perform different functions e.g., forelimbs of humans and wings of birds
• Analogous organs: different origins, but perform similar functions e.g., wings of birds and bats
• Fossils: remains of organisms that once existed on the Earth
• Palaeontology: science dealing with the study of fossils
• Vestigial organs: organs present in the reduced form, having no function
• Human beings (Homo sapiens): evolved from primates in Africa
Chapter 6: Human Eye and the Colorful World• Analogous organs: different origins, but perform similar functions e.g., wings of birds and bats
• Fossils: remains of organisms that once existed on the Earth
• Palaeontology: science dealing with the study of fossils
• Vestigial organs: organs present in the reduced form, having no function
• Human beings (Homo sapiens): evolved from primates in Africa
❖ Important components of the human eye
• Image forms on the retina
• Iris controls the size of the pupil
• Pupil controls the amount of light
• Lens can adjust its focal length. It is called power of accommodation.
• Thickness of the eye can be controlled by ciliary muscles.
• Iris controls the size of the pupil
• Pupil controls the amount of light
• Lens can adjust its focal length. It is called power of accommodation.
• Thickness of the eye can be controlled by ciliary muscles.
❖ Nearest focal distance of lens = 25 cm
❖ Common defects in eye
• Myopia/near-sightedness
Problem: Distant objects cannot be seen clearly
Image is formed in front of the retina
Correction – concave lens
Problem: Distant objects cannot be seen clearly
Image is formed in front of the retina
Correction – concave lens
• Hypermetropia/far-sightedness
Problem: Near objects are not seen clearly
Image formed beyond the retina
Correction – convex lens
Problem: Near objects are not seen clearly
Image formed beyond the retina
Correction – convex lens
• Presbyopia
Problem: Near-focus distance increases with age
Power of accommodation decreases
Correction– bi-focal lens
Problem: Near-focus distance increases with age
Power of accommodation decreases
Correction– bi-focal lens
❖ Prism
.png)
❖ Dispersion
.png)
❖ Atmospheric refraction
• Twinkling of stars – caused by changing air density in the atmosphere
• Early sunrise and delayed sunset – caused by refraction of light through the atmosphere
.png)
• Early sunrise and delayed sunset – caused by refraction of light through the atmosphere
.png)
❖ Scattering: Tyndall effect
Atmospheric particles, smoke, tiny water droplets, suspended particles of dust, and air molecules scatter sunlight. Therefore, the path of light becomes visible.
• Sky is blue- because light near blue wavelength scatters most
• Danger signs are red in colour- because red light scatters least
Chapter 3: How Do Organisms Reproduce• Danger signs are red in colour- because red light scatters least
❖ Reproduction
• Biological process by which a living organism produces an offspring similar to itself
• Information transferred from the parents to the offspring in the form of DNA
• DNA (Deoxyribonucleic acid)- a genetic material found in chromosomes present in the nucleus of a cell
• Two types of reproduction—sexual and asexual
• Information transferred from the parents to the offspring in the form of DNA
• DNA (Deoxyribonucleic acid)- a genetic material found in chromosomes present in the nucleus of a cell
• Two types of reproduction—sexual and asexual
❖ Asexual reproduction
• Does not involve the fusion of gametes
• Requires only one parent
• Offspring’s produced are exact copies of their parents
• Modes of asexual reproduction
• Requires only one parent
• Offspring’s produced are exact copies of their parents
• Modes of asexual reproduction
⚬ Fission- involves cell division or splitting of cells
⚬ Is of two types:
⚬ Is of two types:
◾Binary fission
| Along any plane | e.g., Amoeba
.png) |
| Along single longitudinal plane | e.g., Leishmania.png) |
◾Multiple fission: e.g., Plasmodium
.png)
⚬ Fragmentation- New organisms are formed from fragments of parents. e.g., lichens
⚬ Regeneration- New organisms are formed from body parts. e.g., Planaria
⚬ Regeneration- New organisms are formed from body parts. e.g., Planaria
.png)
⚬ Budding- New individuals form protrusion called buds. e.g., Hydra
.png)
⚬ Vegetative propagation- New plants are formed from the vegetative parts .e.g., Bryophyllum
.png)
⚬ Spore formation- Large number of spores produced in sporangia. e.g., Rhizopus
.png)
❖ Sexual reproduction
• Involves fusion of male and female gametes
• Requires two parents
• Allows more variations in offsprings
• Sexual reproduction in plants
• Requires two parents
• Allows more variations in offsprings
• Sexual reproduction in plants
⚬ Angiosperms- flowering plants
⚬ Parts of flowers– sepals, petals, stamens, and carpels/pistils
⚬ Parts of flowers– sepals, petals, stamens, and carpels/pistils
.png)
◾ Stamens: Male reproductive parts of flowers and consists of anther and filament
◾Carpels: Female reproductive parts of flowers and consists of style, stigma, and ovary
◾Carpels: Female reproductive parts of flowers and consists of style, stigma, and ovary
⚬ Bisexual flowers: Both stamens and carpels are present e.g., hibiscus
⚬ Unisexual flowers: Either stamen or carpel is present e.g., corn
⚬ Pollen released from the bursting of anther, which contains male gametes
⚬ Each ovule contains one egg cell or female gamete
⚬ Pollination: transfer of pollen from the anther of one flower to the stigma of the same or different flower
⚬ Fertilization: Process of fusion of male and female gametes
⚬ After fertilization: zygote = embryo, ovule = seed, ovary = fruit
⚬ Unisexual flowers: Either stamen or carpel is present e.g., corn
⚬ Pollen released from the bursting of anther, which contains male gametes
⚬ Each ovule contains one egg cell or female gamete
⚬ Pollination: transfer of pollen from the anther of one flower to the stigma of the same or different flower
⚬ Fertilization: Process of fusion of male and female gametes
⚬ After fertilization: zygote = embryo, ovule = seed, ovary = fruit
• Sexual reproduction in animals
⚬ Puberty: A period of physical change by which a child’s body becomes an adult’s body and capable of reproduction
⚬ Secondary sex characteristics: The body changes during puberty
⚬ Male reproductive organs: Pair of testes, vas deferens, prostate gland, seminal vesicles
⚬ Testes: Produce sperms and hormone testosterone
⚬ Sperms: Contain male gametes
⚬ Female reproductive organs: Pair of ovaries, pair of oviducts, uterus, and vagina
⚬ Ovaries contain thousands of eggs
⚬ Sperms enter the female body through the vagina
⚬ Fertilization: The process of fusion of the nucleus of the sperm with the ovum to form a zygote
⚬ Zygote divides to form an embryo
⚬ Embryo implanted in the uterus
⚬ Foetus develops inside the mother’s body for nine months
⚬ Menstruation: If the egg is not fertilized, then the uterus lining breaks down and is released in the form of blood and mucous through the vagina
⚬ Sexually transmitted diseases: Infections that get transferred through sexual contact e.g., herpes, HIV-AIDS, syphilis, gonorrhea etc.
⚬ Contraceptive methods help avoid pregnancy: These include natural methods, barrier methods, oral contraceptives, implants, and surgical methods
Chapter 7: Our Environment⚬ Secondary sex characteristics: The body changes during puberty
⚬ Male reproductive organs: Pair of testes, vas deferens, prostate gland, seminal vesicles
⚬ Testes: Produce sperms and hormone testosterone
⚬ Sperms: Contain male gametes
⚬ Female reproductive organs: Pair of ovaries, pair of oviducts, uterus, and vagina
⚬ Ovaries contain thousands of eggs
⚬ Sperms enter the female body through the vagina
⚬ Fertilization: The process of fusion of the nucleus of the sperm with the ovum to form a zygote
⚬ Zygote divides to form an embryo
⚬ Embryo implanted in the uterus
⚬ Foetus develops inside the mother’s body for nine months
⚬ Menstruation: If the egg is not fertilized, then the uterus lining breaks down and is released in the form of blood and mucous through the vagina
⚬ Sexually transmitted diseases: Infections that get transferred through sexual contact e.g., herpes, HIV-AIDS, syphilis, gonorrhea etc.
⚬ Contraceptive methods help avoid pregnancy: These include natural methods, barrier methods, oral contraceptives, implants, and surgical methods
❖ Environment: natural surroundings and external conditions of an organism, which include all living and non-living factors that affect the organism
❖ Organism: is the basic unit of an ecological hierarchy, can be unicellular such as Amoeba and Paramecium or multicellular such as humans
❖ Population: a group of individuals of the same species inhabiting a given geographical area at a particular time and functioning as a unit
❖ Community: includes all individuals of different species living within a certain geographical area
❖ Ecosystem: includes both living and non-living components of an area
• Components of an ecosystem
⚬ Abiotic factors: light, temperature, water, air etc.
⚬ Biotic factors: living organisms
⚬ Autotrophs: organisms that can manufacture their own food from inorganic raw materials, also known as producers
⚬ Heterotrophs: cannot synthesize their own food, are dependent on other organisms
⚬ Herbivores: feed only on plants e.g., deer, horse, sheep etc.
⚬ Carnivores: eat other animals e.g., frog, cat, spider etc.
⚬ Omnivores: feed on both plants and animals e.g. bear, monkey, man etc.
⚬ Decomposers: obtain nutrients by breaking down remains of dead plants and animals, includes some bacteria and fungi
⚬ Biotic factors: living organisms
⚬ Autotrophs: organisms that can manufacture their own food from inorganic raw materials, also known as producers
⚬ Heterotrophs: cannot synthesize their own food, are dependent on other organisms
⚬ Herbivores: feed only on plants e.g., deer, horse, sheep etc.
⚬ Carnivores: eat other animals e.g., frog, cat, spider etc.
⚬ Omnivores: feed on both plants and animals e.g. bear, monkey, man etc.
⚬ Decomposers: obtain nutrients by breaking down remains of dead plants and animals, includes some bacteria and fungi
• Functions of an ecosystem
⚬ Productivity: rate of production of organic matter (food) by producers
⚬ Decomposition: breakdown of organic matter or biomass with the help of decomposers
⚬ Decomposition: breakdown of organic matter or biomass with the help of decomposers
• Energy flow through an ecosystem
⚬ Trophic level: level of species in an ecosystem on the basis of the source of nutrition
⚬ Producers: form the first trophic level, they manufacture food
⚬ trophic levels are connected through food chains
⚬ Food chain: a linear sequence of organisms in which each organism is eaten by the next member in the sequence e.g., plants®grasshopper®frog®snake®eagle
⚬ Food web: interconnected network of food chains
⚬ 10% law of energy transfer: only 10% energy is transferred from a lower trophic level to a higher trophic level, which means that energy keeps on decreasing as one moves up different trophic levels
⚬ Producers: form the first trophic level, they manufacture food
⚬ trophic levels are connected through food chains
⚬ Food chain: a linear sequence of organisms in which each organism is eaten by the next member in the sequence e.g., plants®grasshopper®frog®snake®eagle
⚬ Food web: interconnected network of food chains
⚬ 10% law of energy transfer: only 10% energy is transferred from a lower trophic level to a higher trophic level, which means that energy keeps on decreasing as one moves up different trophic levels
⚬ Biomagnification: increase in the concentration of pollutants or harmful chemicals with each step up in the food chain
• Human influence on the environment
⚬ Global warming: increase in the average temperature of the Earth’s surface
⚬ Greenhouse gases: CO2, CH4, O3, CFCs etc.
⚬ Ozone layer: present in the stratosphere, absorbs ultraviolet radiations. It gets depleted due to an increased concentration of chlorine in the atmosphere -
Cl + O3 → ClO + O2
⚬ Biodegradable wastes: produced mainly from plant and animal sources that can be broken down by living organisms
⚬ Non-biodegradable wastes: includes wastes such as plastic, metals etc., that cannot be broken down by living organisms
Chapter 8: Management of Natural Resources⚬ Greenhouse gases: CO2, CH4, O3, CFCs etc.
⚬ Ozone layer: present in the stratosphere, absorbs ultraviolet radiations. It gets depleted due to an increased concentration of chlorine in the atmosphere -
Cl + O3 → ClO + O2
⚬ Biodegradable wastes: produced mainly from plant and animal sources that can be broken down by living organisms
⚬ Non-biodegradable wastes: includes wastes such as plastic, metals etc., that cannot be broken down by living organisms
❖ Natural resources
• These are the natural substances provided by nature that are considered economically important. Example: soil, air, water etc.
❖ 3R principle to save environment
• Reduce
⚬ It refers to the reduction in the consumption of resources.
⚬ Example - Repairing taps to check water leakages
⚬ Example - Repairing taps to check water leakages
• Recycle
⚬ It means to synthesise or extract useful materials from wastes.
⚬ Example - Plastic, paper, glass, and metals can be extracted from the waste scrap
⚬ Example - Plastic, paper, glass, and metals can be extracted from the waste scrap
• Reuse
⚬ It means using a product again and again.
⚬ Example - Plastic bottles containing jams can be used to store pulses in the kitchen
⚬ Example - Plastic bottles containing jams can be used to store pulses in the kitchen
❖ Need to manage resources
• Resources are in limited supply
• Human population is increasing, so the demand for these resources is also increasing exponentially
• Management of resources should be done with a long-term perspective, so that they can be exploited by the future generations
• The damage caused to the environment while extracting resources should be reduced
• Human population is increasing, so the demand for these resources is also increasing exponentially
• Management of resources should be done with a long-term perspective, so that they can be exploited by the future generations
• The damage caused to the environment while extracting resources should be reduced
❖ Type of Natural Resources
• Forests are biodiversity hotspots as they are homes to large number of plants, animals, and microbes.
❖ Stakeholders in forest resources
• The tribal people living inside and around forests depend on forest resources
⚬ Traditional people played important role in the past in protecting forests.
Example - Amrita Devi Bishnoi sacrificed her life along with 363 other people in 1731 to protect ‘Khjiri’ trees from being cut down in Khejrali village near Jodhpur.
⚬ Products of forests
Example - Amrita Devi Bishnoi sacrificed her life along with 363 other people in 1731 to protect ‘Khjiri’ trees from being cut down in Khejrali village near Jodhpur.
⚬ Products of forests
◾Fire wood
◾Lumber
◾Lac
◾Herbs
◾Honey
◾Fruits
◾Lumber
◾Lac
◾Herbs
◾Honey
◾Fruits
• The forest department of the government
⚬ Owns the land and controls forest resources
⚬ Forest department ignores local knowledge and traditional management practices of the forest
⚬ Vast tracts of the forest is converted into plantations of teak, pine and eucalyptus, which supports little biodiversity.
⚬ Forest department ignores local knowledge and traditional management practices of the forest
⚬ Vast tracts of the forest is converted into plantations of teak, pine and eucalyptus, which supports little biodiversity.
• The industrialists
⚬ Use forest resources in unsustainable manner
⚬ Power lobby which pushes the government, ignoring the local people, for the use of forest resources
⚬ Power lobby which pushes the government, ignoring the local people, for the use of forest resources
• The wildlife enthusiasts
⚬ Not dependent on forests
⚬ Considerable say in forest conservation
⚬ Considerable say in forest conservation
❖ Water resources
• Basic need of life
• Most of the Indian agriculture is dependent on monsoons
• Local people have adopted traditional methods to conserve water
• Traditional water-harvesting systems
• Most of the Indian agriculture is dependent on monsoons
• Local people have adopted traditional methods to conserve water
• Traditional water-harvesting systems
⚬ Khadins and nadis in Rajasthan
⚬ Bandharas and tals in Maharashtra
⚬ Bundhis in Madhya Pradesh and Uttar Pradesh
⚬ Ahars and Pynes in Bihar
⚬ Kulhs in Himachal Pradesh
⚬ Ponds in Jammu
⚬ Eris in Tamil Nadu
⚬ Surangamo in Kerala
⚬ Kattas in Karnataka
⚬ Bandharas and tals in Maharashtra
⚬ Bundhis in Madhya Pradesh and Uttar Pradesh
⚬ Ahars and Pynes in Bihar
⚬ Kulhs in Himachal Pradesh
⚬ Ponds in Jammu
⚬ Eris in Tamil Nadu
⚬ Surangamo in Kerala
⚬ Kattas in Karnataka
❖ Coal and petroleum
• They are non-renewable sources of energy.
• Burning of coal and petroleum releases toxic gases such as carbon monoxide, sulphur dioxide, nitrogen dioxide, and greenhouse gases such as carbon dioxide and methane.
• Use of coal and petroleum can be reduced by using alternate sources of energy and switching over to cleaner biofuels.
• Burning of coal and petroleum releases toxic gases such as carbon monoxide, sulphur dioxide, nitrogen dioxide, and greenhouse gases such as carbon dioxide and methane.
• Use of coal and petroleum can be reduced by using alternate sources of energy and switching over to cleaner biofuels.
❖ Sustainable Management
• Interests of all the stakeholders should be given a proper say.
• Benefits of development should reach each and every individual and all generations.
Chapter 5: Light, reflection and refraction• Benefits of development should reach each and every individual and all generations.
❖ Reflection of light is the change in the path of a light ray upon collision with an interface of two medium.
❖ Laws of reflection:
• i (Angle of incidence) = r (angle of reflection)
• Rays AO, OM and OB lie in the same plane.
.png)
• Rays AO, OM and OB lie in the same plane.
.png)
❖ Terms related to spherical mirrors
• Centre of curvature is the centre of the sphere of which the spherical mirror is a part
.png)
• Pole is the centre of mirror
.png)
• Focus is a point where parallel rays (parallel to the principal axis) meet or appear to meet after reflection
.png)
.png)
• Pole is the centre of mirror
.png)
• Focus is a point where parallel rays (parallel to the principal axis) meet or appear to meet after reflection
.png)
❖ Concave mirror and nature of image formed
• All images are real and inverted, except when the object is between the focus and the pole.
• Image size = object size when the object is at the centre of curvature
• Image size = object size when the object is at the centre of curvature
.png)
• Uses:
| ⚬ Torch reflector ⚬ Search light ⚬ Vehicle headlight |
⚬ Dentist’s mirror ⚬ Shaving mirror |
❖ Convex mirror and nature of image formed
• Virtual image
• Erect image
• All images are diminished
• Uses:
• Erect image
• All images are diminished
• Uses:
⚬ Rear-view mirror
⚬ Security mirror
⚬ Security mirror
❖ Mirror formula:
For concave, f → –ve, for convex, f → +ve
Magnification
❖ Laws of refraction
• AO (incident ray), OB (refracted ray), and MON (normal to the interface) are co-planar.
• = constant (Snell’s law)
.png)
• = constant (Snell’s law)
.png)
❖ Refractive index (R.I.)
• (m of 2 w.r.t. 1)
• Absolute RI when medium I = Vacuum or air
• Speed of light{vacuum} = 3 × 108 m/s
• Absolute RI when medium I = Vacuum or air
• Speed of light{vacuum} = 3 × 108 m/s
| Medium (Optically denser) = m > 1 → | .png) |
| Optically rarer = m < 1 → | .png) |
| In all medium | .png) |
❖ Terms related to spherical mirrors
• Centre of curvature = Centre of the sphere of which the lens surfaces is a part of (Same as Spherical mirror)
• Focus = Where parallel rays meet after refraction (On principal axis = principal focus)
• Focus = Where parallel rays meet after refraction (On principal axis = principal focus)
❖ Convex lens and nature of image formed
• Virtual and erect images – when the object is placed between focus and the optical centre (Magnifying glass)
• Real and inverted image at all po
• Image size = object size when object is at centre of curvature
• Real and inverted image at all po
• Image size = object size when object is at centre of curvature
❖ Concave lens and nature of image formed
• Virtual and erect at all object positions
❖ Lens Formula:
For concave lens f = –ve, convex lens f = +ve
❖ Magnification,
❖ Lens power P (Unit dipotre)
