Chapter 10: Reaching the Age Of Adolescence
❖ Adolescence: The time period when the body undergoes changes to reach reproductive maturity is known as adolescence.
It begins at the age of 10 or 11 and lasts till about 18 or 19 years of age.
❖ Puberty: The various changes that occur in the body during adolescence marks the onset of puberty.
❖ Changes that take place during puberty
❖ Hormones
❖ Role of hormones in other animals
❖ Reproductive phase in humans
❖ Sex determination in babies
❖ Reproductive health
❖ Atmosphere: The layer of air present around the earth. It is composed of 78% of nitrogen, 21% of oxygen, and 1% percent other gases such as carbon dioxide, ozone, water vapour, methane, etc.
❖ Air pollution: The phenomenon of contamination of air with unwanted substances so that it becomes harmful for living organisms and non-living substances.
❖ Acid rain: It is formed when sulphur dioxide and nitrogen dioxide present in air react with water droplets to form nitric and sulphuric acid.
It has caused corrosion of the marble of Taj Mahal.
❖ Green house effect: The trapping up of reflected solar radiations by the earth’s atmosphere and gradual heating up is known as greenhouse effect.
❖ Greenhouse gases: These are the gases, which trap the solar radiations, and prevent them from escaping into the atmosphere.
Example: carbon dioxide, methane, nitrous oxide, and water vapours.
❖ Global warming: The gradual warming up of the earth’s atmosphere by the increased carbon dioxide level.
The CO2 level in atmosphere is increasing due to various human activities such as deforestation and burning of fossil fuels.
❖ Prevention of air pollution:
❖ Water pollution: Mixing of harmful substances in water such as sewage and toxic chemicals so that its physical and chemical properties get altered and it becomes toxic for living organisms.
❖ Water pollutants: Substances that pollute water
❖ Sources of water pollution in Ganga river:
❖ Types of water pollutants
❖ Potable water: Water that is fit for drinking is called potable water.
❖ Methods of obtaining potable water
❖ Prevention of water pollution
❖ All natural objects in the sky are celestial objects.
❖ New Moon day: when moon is not visible
❖ Full Moon day: when full moon is visible
❖ Gap between consecutive new moon day and full moon day is of 15 days. .png) ❖ Rotational period and revolution period of moon are the same (almost 29 days).
❖ Rotational period and revolution period of moon are the same (almost 29 days).
❖ Moon’s surface: Because of lack of atmosphere, one cannot hear any sound on moon.
❖ All stars emit their own light. In day time, stars are not visible because of bright sunlight.
❖ The biggest star that can be seen is the sun.
❖ Stars appear to move from east to west because of earth’s rotation from west to east.
❖ Constellations: Groups of stars of particular shapes
❖ Planets revolve around the sun along definite paths, called orbits.
❖ Time taken by a planet to complete one revolution of its orbit is called revolution period.
❖ Time taken by a planet to rotate about its axis is called period of rotation.
❖ Asteroids
❖ Comets
❖ Meteors & Meteorites
❖ Artificial satellite
❖ Reflection of light is the change in the path of a light ray upon collision with an interface of two medium.
❖ Laws of reflection:
❖ Image formation by a plane mirror
❖ Regular and irregular reflection
❖ Kaleidoscope works on the theory of multiple reflections which forms beautiful patterns.
❖ Sunlight consists of several colours.
❖ Splitting of white light is called dispersion.
❖ Human eye
❖ Impression of an image persists for seconds in our brain.
❖ Vitamin A (raw carrots, broccoli, green vegetables, cod-liver oil, etc.) is necessary for good vision.
❖ Braille system is helpful for visually challenged persons for reading.Chapter 15: Some Natural Phenomena
❖ Objects get charged when rubbed with another material.
❖ There are two kinds of charges, positive and negative.
❖ Electroscope is used to detect whether an object is charged or not. It cannot detect the nature of charge.
❖ The process of transfer of charge from a charged object to Earth is called earthing.
❖ The sudden flow of charge (discharge) from cloud to objects on Earth is called lightening.
❖ Lightening safety
❖ Lightning conductor is the pointed metal rod on top of tall buildings.
❖ It transfers charge from lightening to Earth via conducting wire.
❖ Earthquake is the sudden shaking or trembling of the earth’s surface because of disturbance deep inside the earth’s crust. Earthquakes cannot be predicted.
❖ Causes of Earthquake:
❖ Some liquids are good conductor while some are bad conductors.
❖ The electric current passing through a conducting liquid causes chemical reactions. The resulting effects are called chemical effects of electric current.
❖ The process of depositing a desired metal or material, by means of electricity is called electroplating.Chapter 13: Sound
❖ Vibration – to-and-fro or back-and-forth or up-and-down motion of a body
❖ Musical instruments and their vibrating parts
❖ Audible sound – Human ear can hear sounds having frequency in the range of 20-20,000 Hz.
❖ Noise pollution – Presence of unwanted and excessive sound in the environment.
❖ The force which always opposes relative motion of the two surfaces which are in contact.
❖ Reducing friction
❖ Force is a physical quantity which can change speed, direction or shape of a body.
❖ Resultant of more than one force acting on a body:
❖ Effects of force
❖ Contact forces: when the applier is in contact with the body.
❖ None-contact forces
❖ Pressure → Force per unit area
❖ Water and gas exert pressure on the walls of their container, which varies with depth (or height)
❖ At a given depth, pressure is always the same irrespective of the shape/size of the vessel.
❖ Atmospheric pressure = Weight of the atmosphere per unit areaChapter 1: Crop Production And Management
❖ Crop: Same kinds of plants cultivated on a large scale
❖ Basic crop production practices
❖ Sources of irrigation: Wells, tube wells, ponds, lakes, canal, river, dams etc.
❖ The process of separating grains from the husk in the mixture of threshed chaff is called winnowing.
❖ Small scale storage of grains: It is usually done in jute bags and metallic bins.
❖ Large scale storage of grains: It is done in.
❖ Food from animals - Milk from cow, buffalo, goat, and camel; Meat from chicken, goat, and sheep; Eggs from chicken and ducks.
❖ Animal husbandry - The rearing of animals, which includes feeding, breeding, and disease control on a large scale, is called animal husbandry.Chapter 9: Reproduction in Animals
❖ Reproduction: It is a biological process through which living organisms produce offsprings similar to themselves.
❖ Modes of reproduction: Sexual and asexual reproduction.
❖ Sexual reproduction
❖ Fertilization
❖ Test tube baby: A baby conceived by fertilization that occurs outside mother’s body is called test tube baby.
❖ Development of embryo
❖ Viviparous animals: The animals that give birth to live young ones. Example: cows, dogs, and humans.
❖ Oviparous animals: The animals that lay eggs outside the body. Example: birds, lizards, snakes, and frogs.
❖ Metamorphosis
❖ Asexual reproduction: Involves only a single parent and the new individuals are formed without fusion of gametes.
❖ Two common methods of asexual reproduction
❖ Cloning: The process used to create an exact copy of a cell, tissue or an organism. Dolly, a sheep was the first mammal to be cloned by Ian Wilmut and his colleagues in 1996.Chapter 8: Cell - Structure And Function
❖ Cell: Cells are the basic structural units and the building blocks of living organisms.
❖ Discovery of the cell
❖ Number of cells
❖ Shape of the cells
❖ Size of the cells
❖ Cell structure and functions
❖ Components of the cell
❖ Types of cell
❖ Differences between plant and animal cells
.png)
❖Deforestation: Deforestation is the process of removal of forests for industrial, agricultural, and other purposes.
❖Causes of deforestation
❖Consequences of deforestation
❖Conservation of forests and wildlife
❖Protected areas
❖Red Data Book:
❖Migration
❖Recycling of paper
❖Reforestation
❖Ecosystem: An ecosystem is a natural unit consisting of all plants, animals and micro-organisms in an area functioning together with all of the non-living physical factors of the environment
Chapter 6: Combustion And Flame
❖ Combustion: It is a chemical process in which a substance reacts with oxygen to give off heat and light.
❖ Oxygen (in air) is essential for combustion.
❖ Substances that burn in air are called combustible substances (also called fuels) and those that do not burn in air are non-combustible substances.
❖ Ignition temperature: It is the lowest temperature at which a substance catches fire.
❖ Control of fire:
❖ Inflammable substances → They have very low ignition temperature and can easily catch fire with flame.
❖ Types of combustion: Rapid combustion, spontaneous combustion, and explosion
❖ Zones of candle flame:
.png)
❖ A good fuel is one which
❖ Fuel efficiency: It is expressed in terms of calorific value. The unit is kilojoule per kg.
❖ Calorific value: It is the amount of heat energy produced by complete combustion of 1 kg of a fuel.
❖ Harmful effects of burning of fuels:
❖ In the light of availability of natural resources in nature, they can be broadly classified into exhaustible and inexhaustible natural resources.
❖ Fossil fuels : These are formed from the dead remains of living organisms. Examples: coal, petroleum, and natural gas
❖ Coal: A fossil fuel used to cook food, in railway engines to produce steam, in thermal power stations to generate electricity.
❖ Products of coal: Coke, coal tar, and coal gas
❖ Petroleum: A fossil fuel formed from the dead organisms present in the sea. The process of separating various constituents of petroleum is known as refining.
❖ Natural gas: It is stored under high pressure as compressed natural gas (CNG). It is used as a fuel for transport vehicles because it is a cleaner fuel (less polluting).
❖ Resources such as coal and petroleum are limited. Burning of such fuels is the major cause of air pollution. Therefore, these fuels should be used only when necessary.Chapter 4: Materials - Metals And Non-Metals
❖ Differences in physical properties of metals and non-metals:
❖ Differences in chemical properties of metals and non-metals:
❖ Displacement reactions → A more reactive metal can displace a less reactive metal from their compounds in aqueous solutions.
❖ Uses of metals: In making machinery, automobiles, jewellery, trains, aeroplanes, cooking utensils, etc.
❖ Uses of non-metals: In fertilizers, in water purification process, crackers, etc. Oxygen, a non-metal, is essential for our life as all living beings inhale it during breathing. Chapter 3: Synthetic Fibres And Plastics
❖ Synthetic fibres (or man-made fibres) : Chains of small units joined together (each small unit is a chemical substance).
❖ Types of Synthetic fibres:
❖ Characteristics of synthetic fibres :
❖ Plastics : Polymer like synthetic materials where the arrangement of small units is linear or cross-linked.png) ❖ Plastics can be recycled, reused, coloured, melted, rolled into sheets, or made into wires.
❖ Plastics can be recycled, reused, coloured, melted, rolled into sheets, or made into wires.
❖ Types of plastics :
❖ Characteristics of plastics:
❖ Harmful effects of plastics:
❖ Biodegradable substances: Get decomposed through natural processes such as by the action of bacteria. Examples: paper, peels of vegetables, wood and fruits, etc.
❖ Non-biodegradable substances: Do not get decomposed easily by natural processes. Examples: plastic bags, metals, etc.Chapter 2: Microorganisms
❖Microorganisms: The living organisms that cannot be seen with unaided eye are called microorganisms.
❖Classification of microorganisms: There are five major groups of microorganisms.
❖Importance of microorganisms
❖ Harmful microorganisms – Disease-causing microorganisms are called pathogens.
❖Food preservation
❖Methods of food preservation
• Storage and packing: Dry fruits and vegetables are sealed in air tight packets to prevent microbe attack.
❖Nitrogen cycle: It involves the circulation of nitrogen through living and non-living components of nature.
❖ Adolescence: The time period when the body undergoes changes to reach reproductive maturity is known as adolescence.
It begins at the age of 10 or 11 and lasts till about 18 or 19 years of age.
❖ Puberty: The various changes that occur in the body during adolescence marks the onset of puberty.
❖ Changes that take place during puberty
• Sudden increase in height
⚬ Girls grow faster than boys initially but both reach their maximum height by the age of 18 years.
• Change in body shape
⚬ Boys develop broader shoulders, wider chests and prominent muscles.
⚬ In girls the region below the waist becomes wider
⚬ In girls the region below the waist becomes wider
• Change in the voice pattern
⚬ Voice box or larynx starts growing during puberty.
⚬ It protrudes in males in the neck region and is called Adam’s apple.
⚬ Boys develop deep low-pitched voice while girls develop high-pitched voice.
⚬ It protrudes in males in the neck region and is called Adam’s apple.
⚬ Boys develop deep low-pitched voice while girls develop high-pitched voice.
• Change in activity of sweat and sebaceous glands
⚬ Production of more sweat.
⚬ The oily secretions from sebaceous glands increase. The accumulation of oil and bacterial action leads to acne problems in teenagers.
⚬ The oily secretions from sebaceous glands increase. The accumulation of oil and bacterial action leads to acne problems in teenagers.
• Changes in sex organs
⚬ Testes and penis develop completely in boys. Testes start producing sperms.
⚬ Ovaries develop completely and start producing eggs in girls.
⚬ Ovaries develop completely and start producing eggs in girls.
• Change in intellectual level
⚬ The learning capacity of brain increases.
⚬ Intellectual development takes place during adolescence.
⚬ Intellectual development takes place during adolescence.
• Development of secondary sexual characters
| In boys | In girls |
|
⚬ Appearance of moustaches and beard
⚬ Appearance of hair on chest
⚬ Growth of hair in genital area and other parts of skin
|
⚬ Increase in breast size
⚬ Growth of hair in the pubic regions |
❖ Hormones
• Hormones are chemical secretions that bring about various changes in the body.
• They are produced by endocrine glands.
• These glands release hormones into blood to reach specific target site.
• Production of hormones is under the control of hormones produced from pituitary gland.
• Sex hormones
• They are produced by endocrine glands.
• These glands release hormones into blood to reach specific target site.
• Production of hormones is under the control of hormones produced from pituitary gland.
• Sex hormones
⚬ Male sex hormone is testosterone. It is produced by the testes on the onset of puberty.
⚬ Female sex hormone is called Oestrogen. It is produced by ovaries.
⚬ Female sex hormone is called Oestrogen. It is produced by ovaries.
• Non-sex hormones
Thyroxine
⚬ It is produced by thyroid glands.
⚬ It is required for regulating metabolism in the body.
⚬ Lack of iodine leads to deficiency of thyroxine, which results in a disease called goitre.
⚬ It is required for regulating metabolism in the body.
⚬ Lack of iodine leads to deficiency of thyroxine, which results in a disease called goitre.
Insulin
⚬ It is produced by the pancreas.
⚬ It maintains blood sugar level.
⚬ Deficiency of insulin results in diabetes.
⚬ It maintains blood sugar level.
⚬ Deficiency of insulin results in diabetes.
Adrenalin
⚬ It is secreted by adrenal glands.
⚬ It helps the body to adjust various stress conditions.
⚬ It helps the body to adjust various stress conditions.
Growth hormone
⚬ It is secreted from pituitary glands.
⚬ It is required for proper body growth.
⚬ It is required for proper body growth.
❖ Role of hormones in other animals
• Insect hormone: Ecdysone is an insect hormone that causes metamorphosis in insects.
• Thyroxine: Thyroxine produced by thyroid glands play an important role in the metamorphosis of tadpoles into adults.
• Thyroxine: Thyroxine produced by thyroid glands play an important role in the metamorphosis of tadpoles into adults.
❖ Reproductive phase in humans
• In males, the production of sperm continues throughout the life.
• In females, the reproductive phase starts from 10-12 years and continues till 45-50 years.
• The female reproductive tract undergoes series of cyclic changes, called menstrual cycle, which is of 28 to 30 days.
• One ovum is produced during one cycle.
• The wall of uterus becomes thick to receive fertilized egg.
• If pregnancy does not occur, the unfertilized egg and uterus lining are shed off, which results in bleeding, called menstruation.
• First menstrual flow in a female is called menarche.
• Stoppage of menstruation in females is called menopause.
• In females, the reproductive phase starts from 10-12 years and continues till 45-50 years.
• The female reproductive tract undergoes series of cyclic changes, called menstrual cycle, which is of 28 to 30 days.
• One ovum is produced during one cycle.
• The wall of uterus becomes thick to receive fertilized egg.
• If pregnancy does not occur, the unfertilized egg and uterus lining are shed off, which results in bleeding, called menstruation.
• First menstrual flow in a female is called menarche.
• Stoppage of menstruation in females is called menopause.
❖ Sex determination in babies
• In humans, two chromosomes, called sex chromosomes, are present along with other pairs of chromosomes (autosomes).
• Sex chromosomes are of two types, X and Y.
• Females have two X chromosomes (XX) while males have one X and one Y chromosomes (XY).
• Sex chromosome of the male parent decides the sex of the child.
• When a sperm with X chromosome fuses with an egg containing X chromosome, it leads to the development of a female child.
• When a sperm with Y chromosome fuses with X chromosome of an egg, it leads to the development of a male child.
• Sex chromosomes are of two types, X and Y.
• Females have two X chromosomes (XX) while males have one X and one Y chromosomes (XY).
• Sex chromosome of the male parent decides the sex of the child.
• When a sperm with X chromosome fuses with an egg containing X chromosome, it leads to the development of a female child.
• When a sperm with Y chromosome fuses with X chromosome of an egg, it leads to the development of a male child.
❖ Reproductive health
• Adolescents should have a balanced diet with right proportions of various body nutrients.
• They should maintain cleanliness to prevent bacterial infections.
• They should avoid usage of drugs and alcohols.
• Sharing of syringes during drug abuse leads to spread of HIV virus causes AIDS.
• AIDS is also caused by unsafe sexual contact and from infected mother to her infant through milk.
Chapter 18: Pollution Of Air And Water• They should maintain cleanliness to prevent bacterial infections.
• They should avoid usage of drugs and alcohols.
• Sharing of syringes during drug abuse leads to spread of HIV virus causes AIDS.
• AIDS is also caused by unsafe sexual contact and from infected mother to her infant through milk.
❖ Atmosphere: The layer of air present around the earth. It is composed of 78% of nitrogen, 21% of oxygen, and 1% percent other gases such as carbon dioxide, ozone, water vapour, methane, etc.
❖ Air pollution: The phenomenon of contamination of air with unwanted substances so that it becomes harmful for living organisms and non-living substances.
• Air pollutants: Substances which cause air pollution.
• Sources of air pollution are
• Sources of air pollution are
⚬ Power plants
⚬ Factories
⚬ Automobiles
⚬ Burning of firewood
⚬ Factories
⚬ Automobiles
⚬ Burning of firewood
• Types of air pollutants
⚬ Carbon monoxide: A colourless poisonous gas produced from incomplete burning of fossil fuels. It reduces oxygen carrying capacity of the blood.
⚬ Sulphur dioxide: Produced from combustion of fuels and causes respiratory problems including permanent lungs damage. It causes formation of acid rain.
⚬ Nitrogen dioxide: Produced from incomplete burning of fuels and causes respiratory problems. It causes formation of acid rain.
⚬ Chlorofluorocarbons (CFCs): These are released from refrigerators, air conditioners, and aerosol spray and cause damage to the ozone layer resulting in the formation of ozone hole.
⚬ Suspended particulate matter: These are produced during burning of fossil power plants, mining, steelmaking, and other industrial processes and comprises of tiny particles, which remain suspended in air for a long time.
⚬ Sulphur dioxide: Produced from combustion of fuels and causes respiratory problems including permanent lungs damage. It causes formation of acid rain.
⚬ Nitrogen dioxide: Produced from incomplete burning of fuels and causes respiratory problems. It causes formation of acid rain.
⚬ Chlorofluorocarbons (CFCs): These are released from refrigerators, air conditioners, and aerosol spray and cause damage to the ozone layer resulting in the formation of ozone hole.
⚬ Suspended particulate matter: These are produced during burning of fossil power plants, mining, steelmaking, and other industrial processes and comprises of tiny particles, which remain suspended in air for a long time.
❖ Acid rain: It is formed when sulphur dioxide and nitrogen dioxide present in air react with water droplets to form nitric and sulphuric acid.
It has caused corrosion of the marble of Taj Mahal.
❖ Green house effect: The trapping up of reflected solar radiations by the earth’s atmosphere and gradual heating up is known as greenhouse effect.
❖ Greenhouse gases: These are the gases, which trap the solar radiations, and prevent them from escaping into the atmosphere.
Example: carbon dioxide, methane, nitrous oxide, and water vapours.
❖ Global warming: The gradual warming up of the earth’s atmosphere by the increased carbon dioxide level.
The CO2 level in atmosphere is increasing due to various human activities such as deforestation and burning of fossil fuels.
❖ Prevention of air pollution:
• Use of clear fuels such as CNG, LPG, and unleaded petrol in public and private transport
• Use of renewable sources of energy such as solar, wind, and hydel energy
• Planting more and more trees to prevent pollution
• Prevent burning of dry leaves and use them in composting
• Use of renewable sources of energy such as solar, wind, and hydel energy
• Planting more and more trees to prevent pollution
• Prevent burning of dry leaves and use them in composting
❖ Water pollution: Mixing of harmful substances in water such as sewage and toxic chemicals so that its physical and chemical properties get altered and it becomes toxic for living organisms.
❖ Water pollutants: Substances that pollute water
❖ Sources of water pollution in Ganga river:
• Untreated discharges from textile, paper and sugar mills, and oil refineries
• Disposal of agricultural discharge from near-by fields, which are rich in pesticides and weedicides, into the river
• Flow of untreated domestic sewage into the river
• Cremation of dead bodies into the river
• Immersion of idols of gods and goddesses, flowers, garbage, and polythene bags into the river
• Disposal of agricultural discharge from near-by fields, which are rich in pesticides and weedicides, into the river
• Flow of untreated domestic sewage into the river
• Cremation of dead bodies into the river
• Immersion of idols of gods and goddesses, flowers, garbage, and polythene bags into the river
❖ Types of water pollutants
• Domestic sewage.
• Industrial waste.
• Agricultural waste: Rich in agricultural pesticides and weedicides which causes ground water pollution and an increase in the population of algae in water resulting in eutrophication.
• Industrial waste.
• Agricultural waste: Rich in agricultural pesticides and weedicides which causes ground water pollution and an increase in the population of algae in water resulting in eutrophication.
❖ Potable water: Water that is fit for drinking is called potable water.
❖ Methods of obtaining potable water
• Physical methods
⚬ Boiling of water
⚬ Use of domestic filters such as candle type filter
⚬ Use of domestic filters such as candle type filter
• Chemical methods
⚬ Use of chlorine tablets
❖ Prevention of water pollution
• Proper treatment of industrial waste and domestic waste before its disposal into rivers
• Strict implementation of environmental laws in industrial units
• Reusing water
• Preventing wastage of water
CHAPTER 17: Stars and the Solar System• Strict implementation of environmental laws in industrial units
• Reusing water
• Preventing wastage of water
❖ All natural objects in the sky are celestial objects.
❖ New Moon day: when moon is not visible
❖ Full Moon day: when full moon is visible
❖ Gap between consecutive new moon day and full moon day is of 15 days.
.png)
❖ Moon’s surface: Because of lack of atmosphere, one cannot hear any sound on moon.
❖ All stars emit their own light. In day time, stars are not visible because of bright sunlight.
❖ The biggest star that can be seen is the sun.
❖ Stars appear to move from east to west because of earth’s rotation from west to east.
❖ Constellations: Groups of stars of particular shapes
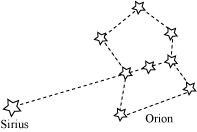 |
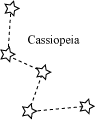 |
.png) |
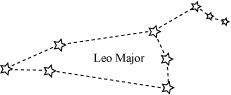 |
❖ Planets revolve around the sun along definite paths, called orbits.
❖ Time taken by a planet to complete one revolution of its orbit is called revolution period.
❖ Time taken by a planet to rotate about its axis is called period of rotation.
| Inner planets | Outer planets |
| Mercury • Nearest planet to the sun • It is seen just before sunrise and just after sunset near horizon. It has no satellite. Venus • Brightest planet planet and nearest to the earth • Also known as morning or evening star • Has no satellite and rotates from east to west (sun rises in the west of Venus) Earth • From space, it appears blue because of 75% water content. Mars • It appears reddish and therefore, is known as red planet |
Jupiter • Largest planet in the solar system • Rotates very fast about its axis and has large numbers of satellites Saturn • Has prominent ring system and large numbers of satellites • Its density is less than water and is the least among the planets Uranus and Neptune • Both have ring system. • Uranus has a tilted rotational axis and appears to roll on its side. • Uranus rotates from east to west similar to Venus. |
❖ Asteroids
• Small rocky objects found in large numbers between Mars and Jupiter
❖ Comets
• Highly elliptical objects
• Have a bright head and long gaseous tail which always directed away from sun
• Halley’s comet appears after every 76 years.
• Have a bright head and long gaseous tail which always directed away from sun
• Halley’s comet appears after every 76 years.
❖ Meteors & Meteorites
• Objects that enter the earth’s atmosphere and burn because of friction with the atmosphere
• Large meteors that reach earth’s surface are called meteorites.
• Large meteors that reach earth’s surface are called meteorites.
❖ Artificial satellite
• Revolves around the earth
• Used for weather forecasting, remote sensing, communication system, etc.
Chapter 16: Light• Used for weather forecasting, remote sensing, communication system, etc.
❖ Reflection of light is the change in the path of a light ray upon collision with an interface of two medium.
❖ Laws of reflection:
• i (angle of incidence) = r (angle of reflection)
• Rays AO, OM and OB lie in the same plane.
.png)
• Rays AO, OM and OB lie in the same plane.
.png)
❖ Image formation by a plane mirror
• Image formed is virtual and of same size as that of object.
• The image is laterally inverted.
.png)
• The image is laterally inverted.
.png)
❖ Regular and irregular reflection
.png) |
|
| Regular reflection | Irregular reflection |
❖ Kaleidoscope works on the theory of multiple reflections which forms beautiful patterns.
❖ Sunlight consists of several colours.
❖ Splitting of white light is called dispersion.
❖ Human eye
• The image forms on retina.
• Iris controls the size of pupil.
• Pupil controls the amount of light.
• Optic nerves sense the image and send it to the brain.
• The junction of optic nerve and the retina is called blind spot.
• Iris controls the size of pupil.
• Pupil controls the amount of light.
• Optic nerves sense the image and send it to the brain.
• The junction of optic nerve and the retina is called blind spot.
❖ Impression of an image persists for seconds in our brain.
❖ Vitamin A (raw carrots, broccoli, green vegetables, cod-liver oil, etc.) is necessary for good vision.
❖ Braille system is helpful for visually challenged persons for reading.Chapter 15: Some Natural Phenomena
❖ Objects get charged when rubbed with another material.
| Objects that get charged | Material used for rubbing |
| Refill | Polythene, woollen cloth |
| Balloon | Polythene, woollen cloth, dry hair |
| Eraser | Wool |
| Steel spoon | Polythene, woollen cloth |
| Ebonite comb | Dry hair, silk cloth |
| Glass rod | Woollen cloth, silk cloth |
❖ There are two kinds of charges, positive and negative.
• Like charges repel each other.
• Opposite charges attract each other.
• Charge generated by rubbing is static.
• When charges move they constitute an electric current.
• Opposite charges attract each other.
• Charge generated by rubbing is static.
• When charges move they constitute an electric current.
❖ Electroscope is used to detect whether an object is charged or not. It cannot detect the nature of charge.
❖ The process of transfer of charge from a charged object to Earth is called earthing.
❖ The sudden flow of charge (discharge) from cloud to objects on Earth is called lightening.
❖ Lightening safety
• Safe places → inside building, inside car/bus, any fully covered place
• Unsafe places → open spaces, beneath tall trees, any elevated place
• Using an umbrella is not safe during lightning.
• During lightning, one should avoid using electrical appliances (should be unplugged) and running water.
• Unsafe places → open spaces, beneath tall trees, any elevated place
• Using an umbrella is not safe during lightning.
• During lightning, one should avoid using electrical appliances (should be unplugged) and running water.
❖ Lightning conductor is the pointed metal rod on top of tall buildings.
❖ It transfers charge from lightening to Earth via conducting wire.
❖ Earthquake is the sudden shaking or trembling of the earth’s surface because of disturbance deep inside the earth’s crust. Earthquakes cannot be predicted.
❖ Causes of Earthquake:
• The uppermost layer of the earth (crust) is fragmented and each fragment is called plate.
• Movements of earth’s plates cause Earthquake. Plate boundaries are called seismic zones or fault zones.
• Earthquake may also occur because of volcanic eruption.
• Power of earthquake is measured on Richter scale.
Chapter 14: Chemical Effects of Electric Current• Movements of earth’s plates cause Earthquake. Plate boundaries are called seismic zones or fault zones.
• Earthquake may also occur because of volcanic eruption.
• Power of earthquake is measured on Richter scale.
❖ Some liquids are good conductor while some are bad conductors.
• Conductivity can be tested by using given set-ups.
.png)
.png)
• The bulb will glow or the magnetic needle will show deflection if the liquid in the beaker is a good conductor of electricity.
• Greater the deflection of needle or brighter the light, better is the conductivity of the liquid.
• Classification of some liquids on the basis of their conductivity.
• Greater the deflection of needle or brighter the light, better is the conductivity of the liquid.
• Classification of some liquids on the basis of their conductivity.
| Good conductor | Poor conductor |
| Lemon juice | Milk |
| Vinegar | Distilled water |
| Acid solutions | Honey |
| Basic solutions | Vegetable oil |
| Salty water (tap water, sea water) |
Kerosene |
❖ The electric current passing through a conducting liquid causes chemical reactions. The resulting effects are called chemical effects of electric current.
❖ The process of depositing a desired metal or material, by means of electricity is called electroplating.Chapter 13: Sound
❖ Sound is a form of energy which produces sensation of hearing.
❖ Vibrating body produces sound.
❖ Vibrating body produces sound.
❖ Vibration – to-and-fro or back-and-forth or up-and-down motion of a body
❖ Musical instruments and their vibrating parts
| Musical instrument | Vibrating part producing sound |
| Veena | Stretched string |
| Tabla | Stretched membrane |
| Flute | Air column |
❖ Sound requires a medium to propagate (solid, liquid, or gas).
❖ Sound cannot travel through vacuum.
❖ In humans, sound is produced by larynx (voice-box).
❖ In human ear, the eardrum vibrates and passes vibration to the inner ear
❖ Frequency – Number of oscillations per unit time. [Unit→hertz = (Hz)]
• Pitch or shrillness depends on frequency.
• Higher frequency → higher pitch
• Higher frequency → higher pitch
❖ Loudness of sound:
• It is measured in decibel (dB).
• It depends on amplitude.
• Higher amplitude → louder sound
• It depends on amplitude.
• Higher amplitude → louder sound
❖ Audible sound – Human ear can hear sounds having frequency in the range of 20-20,000 Hz.
❖ Noise pollution – Presence of unwanted and excessive sound in the environment.
• It can be reduced by plantation on roadside.
Chapter 12: Friction❖ The force which always opposes relative motion of the two surfaces which are in contact.
❖ Cause of friction - the irregularities on the two surfaces in contact lock into one another.
❖ Factors affecting friction• Nature of surfaces – Smooth surfaces → less friction
• Rough surfaces → greater friction
• How hard an object is pressed – Greater pressing force → Greater friction
• Mass of object – Greater mass → Greater friction
• Sliding friction < Static friction
• Rolling friction < Sliding friction
• Rough surfaces → greater friction
• How hard an object is pressed – Greater pressing force → Greater friction
• Mass of object – Greater mass → Greater friction
• Sliding friction < Static friction
• Rolling friction < Sliding friction
❖ Reducing friction
• Lubrication – Powder on carom board, oil in machine
• Wheel – Wheels reduce friction (because rolling friction < sliding friction).
• In many machines, friction is reduced by using ball-bearing.
• Shoe soles and tires are threaded to increase friction for a better grip.
• In many machines, friction is reduced by using ball-bearing.
• Shoe soles and tires are threaded to increase friction for a better grip.
❖ The friction between layers of fluid or between a solid object and fluid is called fluid friction.
❖ Fluid friction between an object and fluid depends upon:
• Speed of the object relative to the fluid
• Shape and size of the object
• Nature of fluid
• Shape and size of the object
• Nature of fluid
❖ Fluid friction is minimised by giving suitable shapes to vehicles moving through fluids.
❖ Fluid friction is also called dragChapter 11: Force and Pressure❖ Force is a physical quantity which can change speed, direction or shape of a body.
• Push→ When an object is moving away from the applier of force
• Pull→ When an object is moving towards the applier of force
• Pull→ When an object is moving towards the applier of force
❖ Resultant of more than one force acting on a body:
• Forces are applied in the same direction → Addition, direction same as the forces
• Force applied in the opposite direction → Subtraction, direction same as of the greater force
• Force applied in the opposite direction → Subtraction, direction same as of the greater force
❖ Effects of force
• Force can change the state of motion.
• Force can change the shape or size of an object.
• Force can change the shape or size of an object.
❖ Contact forces: when the applier is in contact with the body.
• Muscular force – It involves the action of muscles.
• Friction – The force which always opposes the relative motion of two bodies in contact.
• Friction – The force which always opposes the relative motion of two bodies in contact.
❖ None-contact forces
• Magnetic force – Force active between two magnets or one magnet and one non-magnet
• Electrostatic force – Force of attraction/repulsion between electric charges
• Gravitational force – Force of attraction between any two bodies in the universe
• Electrostatic force – Force of attraction/repulsion between electric charges
• Gravitational force – Force of attraction between any two bodies in the universe
❖ Pressure → Force per unit area
•
❖ Water and gas exert pressure on the walls of their container, which varies with depth (or height)
❖ At a given depth, pressure is always the same irrespective of the shape/size of the vessel.
❖ Atmospheric pressure = Weight of the atmosphere per unit areaChapter 1: Crop Production And Management
❖ Crop: Same kinds of plants cultivated on a large scale
| Types | Season | Examples |
| Kharif crops | Sown in rainy season (June to September). | soyabean, paddy, maize |
| Rabi crops | grown during winter season (October to March) | wheat, gram, pea |
❖ Basic crop production practices
| Practices | Process | Tools |
| Preparation of soil | Loosening and upturning of the soil. This process is known as tilling or ploughing. | • Plough • Hoe • Cultivator |
| Sowing | Placing of seeds of a crop in the soil is called sowing. |
• Funnel shaped traditional tool driven by animals • Seed Drills |
| Adding manure and fertilizers | Adding nutrients to the soil | Manures - Prepared from decomposed animals and plant waste. Fertilizers Commercially available inorganic salts rich in plant nutrients |
| Irrigation | Supplying of water to plants at various intervals is called irrigation. |
• Moat (pulley system) • Chain pump • Dhekli • Rahat (chain system) • Sprinkler system • Drip system |
| Protection from weeds (weeding) | Removal of unwanted plants from the field that compete with crops for space, water and nutrients | • Manually by hands • Seed drills • Khurpi • Weedicides like 2,4-D |
| Harvesting | The process of cutting of mature crops from the field is called harvesting | • Sickle • Combine |
| Threshing | The process of removing grains from chaff is called threshing. | Combine |
| Storage | The process of keeping seeds safe from spoilage due to moisture, insects, rats, and microorganisms for a long time is called storage | • Jute bags and metallic bins for small scale storage • Silos and granaries for large scale storage |
❖ Sources of irrigation: Wells, tube wells, ponds, lakes, canal, river, dams etc.
❖ The process of separating grains from the husk in the mixture of threshed chaff is called winnowing.
❖ Small scale storage of grains: It is usually done in jute bags and metallic bins.
❖ Large scale storage of grains: It is done in.
❖ Food from animals - Milk from cow, buffalo, goat, and camel; Meat from chicken, goat, and sheep; Eggs from chicken and ducks.
❖ Animal husbandry - The rearing of animals, which includes feeding, breeding, and disease control on a large scale, is called animal husbandry.Chapter 9: Reproduction in Animals
❖ Reproduction: It is a biological process through which living organisms produce offsprings similar to themselves.
❖ Modes of reproduction: Sexual and asexual reproduction.
❖ Sexual reproduction
• Involves the fusion of male and female gametes.
• Male gametes are called sperms and female gametes are called ova.
• Male reproductive system: Consists of testis, sperm duct and penis.
• Male gametes are called sperms and female gametes are called ova.
• Male reproductive system: Consists of testis, sperm duct and penis.
⚬ Testis is involved in sperm production.
⚬ Sperm contains three parts: Head, middle piece and tail.
⚬ Sperm contains three parts: Head, middle piece and tail.
• Female reproductive system: Consists of ovaries, oviduct and uterus.
⚬ Ovaries produce ova or eggs.
⚬ A single matured egg is released from ovum into oviduct every month.
⚬ A single matured egg is released from ovum into oviduct every month.
❖ Fertilization
• The process of fusion of male and female gametes (egg and sperm) to form zygote is known as fertilization. There are two types of fertilisation;
⚬ Internal fertilization: In this, the fusion of sperm and egg takes place inside the female body. For example, cows, dogs and humans.
⚬ External fertilization: In this, the fusion of sperm and eggs takes place outside the female body in a surrounding medium, generally water. It occurs in frogs, fishes, starfish, etc.
⚬ External fertilization: In this, the fusion of sperm and eggs takes place outside the female body in a surrounding medium, generally water. It occurs in frogs, fishes, starfish, etc.
❖ Test tube baby: A baby conceived by fertilization that occurs outside mother’s body is called test tube baby.
❖ Development of embryo
• The zygote repeatedly divides to form a ball of cells.
• The ball of cells then starts differentiating into tissues and organs. At this stage, it is called embryo.
• Embryo gets attached to the wall of the uterus and develops various body parts such as hands and legs.
• Foetus is a stage of embryo that shows main recognizable feature of mature organism.
• Foetus develops for nine months inside the mother’s womb and is finally delivered.
• The ball of cells then starts differentiating into tissues and organs. At this stage, it is called embryo.
• Embryo gets attached to the wall of the uterus and develops various body parts such as hands and legs.
• Foetus is a stage of embryo that shows main recognizable feature of mature organism.
• Foetus develops for nine months inside the mother’s womb and is finally delivered.
❖ Viviparous animals: The animals that give birth to live young ones. Example: cows, dogs, and humans.
❖ Oviparous animals: The animals that lay eggs outside the body. Example: birds, lizards, snakes, and frogs.
❖ Metamorphosis
• The biological process of transformation of larva into adult is known as metamorphosis.
• The life cycle of frog consists of the following stages.
Egg → Tadpole (larva) → Adult
• The life cycle of a silk worm consists of the following stages.
Egg → Larva → Pupa → Adult
• The life cycle of frog consists of the following stages.
Egg → Tadpole (larva) → Adult
• The life cycle of a silk worm consists of the following stages.
Egg → Larva → Pupa → Adult
❖ Asexual reproduction: Involves only a single parent and the new individuals are formed without fusion of gametes.
❖ Two common methods of asexual reproduction
• Budding
⚬ Budding involves the formation of new individual from the bulging of parent body.
⚬ This phenomenon is very common in plants, fungi and animals such as Hydra and yeast.
⚬ This phenomenon is very common in plants, fungi and animals such as Hydra and yeast.
• Fission
⚬ Binary fission is the type of asexual reproduction that occurs in Amoeba.
⚬ It is a type of asexual reproduction in which a single cell divides into two halves.
⚬ It is a type of asexual reproduction in which a single cell divides into two halves.
⚬
.png)
.png)
❖ Cloning: The process used to create an exact copy of a cell, tissue or an organism. Dolly, a sheep was the first mammal to be cloned by Ian Wilmut and his colleagues in 1996.Chapter 8: Cell - Structure And Function
❖ Cell: Cells are the basic structural units and the building blocks of living organisms.
❖ Discovery of the cell
• Cell was first discovered by Robert Hooke in 1665 after observing a piece of cork under a magnifying device.
• Hooke coined the term “cell”.
• Hooke coined the term “cell”.
❖ Number of cells
• Organisms made of only a single cell are called unicellular organisms; for example: Amoeba and Paramecium
• Single cell in these organisms performs all the basic functions such as digestion, respiration, and excretion.
• Organisms made up of more than one cells are called multicellular organisms. For example: Humans, cow, etc.
• In these organisms, the cells show division of labour as particular set of cells are involved in performing a specific body function.
• Single cell in these organisms performs all the basic functions such as digestion, respiration, and excretion.
• Organisms made up of more than one cells are called multicellular organisms. For example: Humans, cow, etc.
• In these organisms, the cells show division of labour as particular set of cells are involved in performing a specific body function.
❖ Shape of the cells
• Some cells such as Amoeba have no definite shape.
• The human red blood cell (RBC) is spherical-shaped.
• The muscle cells in humans are spindle-shaped.
• The human nerve cells have elongated branched structure.
• In plants and bacteria, the cell is enclosed in a protective covering called cell wall, which gives shape and rigidity to the cells.
• The human red blood cell (RBC) is spherical-shaped.
• The muscle cells in humans are spindle-shaped.
• The human nerve cells have elongated branched structure.
• In plants and bacteria, the cell is enclosed in a protective covering called cell wall, which gives shape and rigidity to the cells.
❖ Size of the cells
• The smallest cell is 0.1 to 0.5 μm in bacteria.
• The largest cell is the egg of an ostrich which is about 170 mm ´ 130 mm in size.
• The largest cell is the egg of an ostrich which is about 170 mm ´ 130 mm in size.
❖ Cell structure and functions
• In multicellular organisms, each organ system is made up of several organs.
• Organs are further made up of tissues.
• Tissues are groups of similar cells performing a specific function
• Organs are further made up of tissues.
• Tissues are groups of similar cells performing a specific function
❖ Components of the cell
• Cell membrane
⚬ It is the protective layer that surrounds the cell.
⚬ Cell membrane selectively allows the entry of only some substances and prevents the movement of other materials. Hence, it checks the transport of substances in and out of the cell.
⚬ Cell membrane selectively allows the entry of only some substances and prevents the movement of other materials. Hence, it checks the transport of substances in and out of the cell.
• Cell wall
⚬ An extra protective covering of a polysaccharide, cellulose is present In plants.
• Cytoplasm
⚬ A jelly-like substance present between cell membrane and nucleus.
⚬ It contains various cell organelles such as mitochondria, golgi bodies, lysosomes etc.
⚬ It contains various cell organelles such as mitochondria, golgi bodies, lysosomes etc.
• Nucleus
⚬ It is surrounded by porous nuclear membrane.
⚬ It contains spherical body called nucleolus.
⚬ It also contains thread-like structures called chromosomes.
⚬ Chromosomes are the structures that carry genes and play an important role in inheritance.
⚬ Genes are the structural and functional unit of inheritance.
⚬ The entire living substance in a cell is known as protoplast.
⚬ It contains spherical body called nucleolus.
⚬ It also contains thread-like structures called chromosomes.
⚬ Chromosomes are the structures that carry genes and play an important role in inheritance.
⚬ Genes are the structural and functional unit of inheritance.
⚬ The entire living substance in a cell is known as protoplast.
• Vacuoles
⚬ Vacuoles are fluid-filled membrane-bound structures in the cell.
⚬ In plant cells, a single large vacuole is present.
⚬ In animal cells, numerous small vacuoles are present.
⚬ In plant cells, a single large vacuole is present.
⚬ In animal cells, numerous small vacuoles are present.
• Plastids
⚬ They are present only in plant cells.
⚬ Plastid that contains green colour pigment called chlorophyll is known as chloroplasts. It is the chlorophyll that gives green colour to the leaves.
⚬ Chloroplast traps solar energy and utilizes this energy to manufacture food for the plant.
⚬ Plastid that contains green colour pigment called chlorophyll is known as chloroplasts. It is the chlorophyll that gives green colour to the leaves.
⚬ Chloroplast traps solar energy and utilizes this energy to manufacture food for the plant.
❖ Types of cell
• Prokaryotic cells - Cells having nuclear material without well defined nuclear membrane. For example - bacteria, blue green algae
• Eukaryotic cells - Cells having nucleus with well defined nuclear membrane. For example - plant and animal cells
• Eukaryotic cells - Cells having nucleus with well defined nuclear membrane. For example - plant and animal cells
❖ Differences between plant and animal cells
| Cell organelle | Plant Cell | Animal Cell |
| Cell Wall | Present | Absent |
| Cell Membrane | Present | Present |
| Nucleus | Present | Present |
| Nuclear Membrane | Present | Present |
| Plastids | Present | Absent |
| Cytoplasm | Present | Present |
| Vacuoles | Present | Present |
• Plant cell
.png)
• Animal cells
.png)
Chapter 7: Conservation Of Plants And Animals.png)
❖Deforestation: Deforestation is the process of removal of forests for industrial, agricultural, and other purposes.
❖Causes of deforestation
• Natural causes
⚬ Forest fire
⚬ Severe droughts
⚬ Severe droughts
• Man-made causes
⚬ Using land for agricultural purposes
⚬ Rapid urbanization
⚬ Procurement of wood for fuel and furniture
⚬ Rapid urbanization
⚬ Procurement of wood for fuel and furniture
❖Consequences of deforestation
• Increase in the level of carbon dioxide in atmosphere, which leads to global warming
• Lowering of ground water levels
• Increase in pollution levels and temperature
• Decrease in fertility of soil and amount of rainfall
• Increase in frequency of droughts and floods
• Conversion of fertile land into deserts (desertification)
• Lowering of ground water levels
• Increase in pollution levels and temperature
• Decrease in fertility of soil and amount of rainfall
• Increase in frequency of droughts and floods
• Conversion of fertile land into deserts (desertification)
❖Conservation of forests and wildlife
• Biodiversity: It is the number and variety of life forms such as plants, animals and microorganisms in an area.
• Forests help in maintaining the delicate balance of nature.
• Animals living in forests are called wild animals.
• Flora: The plants found in a particular area.
• Fauna: The animals found in a particular area.
• Endemic species: Species of plants and animals that are found only in a particular area, are called endemic species.
• Species: It is a group of population which are capable of interbreeding.
• Endangered animals: The animals, whose numbers are diminishing to a level that they might face extinction. For example: tiger, lion, and elephants
• Project tiger was launched to protect endangered tigers in their natural habitat.
• The flora and fauna of a particular habitat can be protected through special protected areas.
• Forests help in maintaining the delicate balance of nature.
• Animals living in forests are called wild animals.
• Flora: The plants found in a particular area.
• Fauna: The animals found in a particular area.
• Endemic species: Species of plants and animals that are found only in a particular area, are called endemic species.
• Species: It is a group of population which are capable of interbreeding.
• Endangered animals: The animals, whose numbers are diminishing to a level that they might face extinction. For example: tiger, lion, and elephants
• Project tiger was launched to protect endangered tigers in their natural habitat.
• The flora and fauna of a particular habitat can be protected through special protected areas.
❖Protected areas
• Wildlife sanctuary: It is the place where wild animals are protected from hunting and damage to their habitat.
For example: Mudumalai wildlife sanctuary in Tamil Nadu, Chilika bird sanctuary in Orissa, etc.
• National parks: These are the areas reserved for wildlife where they can freely use the habitats and natural resources. For example:
Ranthambore national park in Rajasthan, Kanha national park in Madhya Pradesh, etc. Satpura national park is the first reserve forest of India.
The finest Indian teak is found in this forest.
• Biosphere reserves: It is a large protected land for conservation of wild life, plant and animals resources, and the traditional life of the tribals living in the area.
For example: Pachmarhi Biosphere reserve and Nilgiri Biosphere reserve in India
For example: Mudumalai wildlife sanctuary in Tamil Nadu, Chilika bird sanctuary in Orissa, etc.
• National parks: These are the areas reserved for wildlife where they can freely use the habitats and natural resources. For example:
Ranthambore national park in Rajasthan, Kanha national park in Madhya Pradesh, etc. Satpura national park is the first reserve forest of India.
The finest Indian teak is found in this forest.
• Biosphere reserves: It is a large protected land for conservation of wild life, plant and animals resources, and the traditional life of the tribals living in the area.
For example: Pachmarhi Biosphere reserve and Nilgiri Biosphere reserve in India
❖Red Data Book:
• It is the source book maintained by IUCN (International Union for Conservation of Nature and Natural resources).
It keeps a track record of various endangered species of plants and animals.
It keeps a track record of various endangered species of plants and animals.
❖Migration
• It is the movement of birds and animals from their original habitat to other places at a particular time every year.
• Numerous migratory birds including ducks, geese, sandpipers, and cranes fly to India every year.
• Migration by birds every year occurs due to
• Numerous migratory birds including ducks, geese, sandpipers, and cranes fly to India every year.
• Migration by birds every year occurs due to
⚬ change in climatic conditions in their original habitat
⚬ lack of food availability in their original habitats during winters
⚬ lack of food availability in their original habitats during winters
❖Recycling of paper
• One ton of paper is made from about seventeen fully-grown trees.
• Papers should be recycled and reused to conserve forest.
• Each paper can be recycled three to seven times.
• Recycling of paper saves energy and water.
• It prevents the release of harmful chemicals (used during paper manufacturing) in nature.
• Papers should be recycled and reused to conserve forest.
• Each paper can be recycled three to seven times.
• Recycling of paper saves energy and water.
• It prevents the release of harmful chemicals (used during paper manufacturing) in nature.
❖Reforestation
• Restocking of destroyed forests by planting new trees is called reforestation.
• It helps in checking environmental degradation.
• It helps in checking environmental degradation.
❖Ecosystem: An ecosystem is a natural unit consisting of all plants, animals and micro-organisms in an area functioning together with all of the non-living physical factors of the environment
Chapter 6: Combustion And Flame
❖ Combustion: It is a chemical process in which a substance reacts with oxygen to give off heat and light.
❖ Oxygen (in air) is essential for combustion.
❖ Substances that burn in air are called combustible substances (also called fuels) and those that do not burn in air are non-combustible substances.
❖ Ignition temperature: It is the lowest temperature at which a substance catches fire.
❖ Control of fire:
• Water is commonly used to extinguish fire. It is not suitable for fires involving oil, petrol, and electrical equipments.
• For fires involving oil, petrol, and electrical equipments, carbon dioxide is the best extinguisher.
• For fires involving oil, petrol, and electrical equipments, carbon dioxide is the best extinguisher.
❖ Inflammable substances → They have very low ignition temperature and can easily catch fire with flame.
❖ Types of combustion: Rapid combustion, spontaneous combustion, and explosion
❖ Zones of candle flame:
.png)
| Zone | Temperature | Colour | Combustion |
| Outermost (Luminous) | Maximum | Blue | Complete |
| Middle | Moderate | Yellow | Partial |
| Innermost (Non-luminous) | Minimum | Black | Unburnt carbon particles |
❖ A good fuel is one which
• is cheap
• is readily available
• burns easily in air at a moderate rate
• produces large amount of heat
• does not leave behind any undesirable substances
• is readily available
• burns easily in air at a moderate rate
• produces large amount of heat
• does not leave behind any undesirable substances
❖ Fuel efficiency: It is expressed in terms of calorific value. The unit is kilojoule per kg.
❖ Calorific value: It is the amount of heat energy produced by complete combustion of 1 kg of a fuel.
❖ Harmful effects of burning of fuels:
• Burning of fuels releases unburnt fine carbon particles, which cause respiratory diseases such as asthma.
• Incomplete combustion of fuels produces carbon monoxide gas (poisonous gas).
• Increased percentage of carbon dioxide in the atmosphere causes global warming.
• Oxides of sulphur and nitrogen produced by burning of fuels (coal, diesel, petrol, etc.) cause acid rain, which is harmful for crops, buildings, and soil.
Chapter 5: Coal And Petroleum• Incomplete combustion of fuels produces carbon monoxide gas (poisonous gas).
• Increased percentage of carbon dioxide in the atmosphere causes global warming.
• Oxides of sulphur and nitrogen produced by burning of fuels (coal, diesel, petrol, etc.) cause acid rain, which is harmful for crops, buildings, and soil.
❖ In the light of availability of natural resources in nature, they can be broadly classified into exhaustible and inexhaustible natural resources.
• Inexhaustible natural resources: They are present in unlimited quantity in nature. Examples: sunlight, air
• Exhaustible natural resources: The amount of these resources in nature is limited. Examples: coal, petroleum, natural gas, etc.
• Exhaustible natural resources: The amount of these resources in nature is limited. Examples: coal, petroleum, natural gas, etc.
❖ Fossil fuels : These are formed from the dead remains of living organisms. Examples: coal, petroleum, and natural gas
❖ Coal: A fossil fuel used to cook food, in railway engines to produce steam, in thermal power stations to generate electricity.
❖ Products of coal: Coke, coal tar, and coal gas
• Coke: It is the pure form of carbon and is used in the extraction of steel and many other metals.
• Coal tar: It is the mixture of about 200 substances. The products obtained from coal tar are used as starting materials for dyes, drugs, paints, perfumes, etc.
• Coal gas: It is obtained during the processing of coal to obtain coke.
• Coal tar: It is the mixture of about 200 substances. The products obtained from coal tar are used as starting materials for dyes, drugs, paints, perfumes, etc.
• Coal gas: It is obtained during the processing of coal to obtain coke.
❖ Petroleum: A fossil fuel formed from the dead organisms present in the sea. The process of separating various constituents of petroleum is known as refining.
• Products of petroleum: Petrol, diesel, kerosene, paraffin wax, lubricating oil, and petroleum gas
| Constituents of Petroleum | Uses |
| LPG | As fuel for home and industry |
| Petrol | As motor fuel, aviation fuel |
| Paraffin wax | Candle, vaseline, etc. |
| Diesel | As fuel for heavy motor vehicles |
| Kerosene | As fuel for stoves, lamps |
❖ Natural gas: It is stored under high pressure as compressed natural gas (CNG). It is used as a fuel for transport vehicles because it is a cleaner fuel (less polluting).
❖ Resources such as coal and petroleum are limited. Burning of such fuels is the major cause of air pollution. Therefore, these fuels should be used only when necessary.Chapter 4: Materials - Metals And Non-Metals
❖ Differences in physical properties of metals and non-metals:
| Metals | Non-metals |
| Generally, found in solid states (Exception: Mercury is a liquid metal) | Generally, found in liquid and gaseous states. |
| Generally, these are hard and lustrous (Exception: Sodium and potassium are soft and can be cut with knife). | These are soft and have no lustre. |
| These are malleable and ductile. | These do not show such properties. |
| These are sonorous (produce ringing sound when struck). | These are not sonorous. |
| These are good conductors of heat and electricity. | These are poor conductors of heat and electricity. |
❖ Differences in chemical properties of metals and non-metals:
| Metals | Non-metals |
| These react with oxygen to produce metal oxides, which are basic in nature. | These react with oxygen to form non-metallic oxides, which are acidic in nature. |
| Some metals react with water to produce metal hydroxides and hydrogen. | These do not react with water. |
| These react with acids to produce metal salts and hydrogen gas. | These do not react with acids. |
| Some metals react with bases to produce hydrogen gas. | Reactions of non-metals with bases are complex. |
❖ Displacement reactions → A more reactive metal can displace a less reactive metal from their compounds in aqueous solutions.
• Example:
❖ Uses of metals: In making machinery, automobiles, jewellery, trains, aeroplanes, cooking utensils, etc.
❖ Uses of non-metals: In fertilizers, in water purification process, crackers, etc. Oxygen, a non-metal, is essential for our life as all living beings inhale it during breathing. Chapter 3: Synthetic Fibres And Plastics
❖ Synthetic fibres (or man-made fibres) : Chains of small units joined together (each small unit is a chemical substance).
❖ Types of Synthetic fibres:
• Rayon (or artificial silk): Mixed with cotton to make bed sheets or mixed with wool to make carpets.
• Nylon: Strong, elastic, light and used for making clothes, parachutes and ropes for rock climbing.
• Polyester: Remains crisp and is easy to wash. Terylene (used for making dress materials) and PET (used for making utensils, films, wires and bottles) are two well known polyesters.
• Nylon: Strong, elastic, light and used for making clothes, parachutes and ropes for rock climbing.
• Polyester: Remains crisp and is easy to wash. Terylene (used for making dress materials) and PET (used for making utensils, films, wires and bottles) are two well known polyesters.
• Acrylic: Used for making sweaters, shawls and blankets.
❖ Characteristics of synthetic fibres :
• Dry up quickly
• Durable
• Less expensive
• Readily available
• Easy to maintain
• Durable
• Less expensive
• Readily available
• Easy to maintain
❖ Plastics : Polymer like synthetic materials where the arrangement of small units is linear or cross-linked
.png)
❖ Types of plastics :
• Thermoplastics: Get deformed easily on heating and can be bent easily. Examples: polythene and PVC
• Thermosetting plastics: If moulded once, cannot be softened by heating. Examples: bakelite and melamine
• Thermosetting plastics: If moulded once, cannot be softened by heating. Examples: bakelite and melamine
❖ Characteristics of plastics:
• They are non-reactive.
• They are light, strong, and durable.
• They are poor conductors of heat and electricity.
• They are light, strong, and durable.
• They are poor conductors of heat and electricity.
❖ Harmful effects of plastics:
• They cause environment pollution. To minimise the hazard, 4R principle (Reduce, Reuse, Recycle, and Recover) is used.
❖ Biodegradable substances: Get decomposed through natural processes such as by the action of bacteria. Examples: paper, peels of vegetables, wood and fruits, etc.
❖ Non-biodegradable substances: Do not get decomposed easily by natural processes. Examples: plastic bags, metals, etc.Chapter 2: Microorganisms
❖Microorganisms: The living organisms that cannot be seen with unaided eye are called microorganisms.
❖Classification of microorganisms: There are five major groups of microorganisms.
| Microorganisms | Discripion |
| Bacteria | • Single-celled organisms • Found in wide range of habitats ranging from glaciers to deserts and hot springs • Example – curd bacteria (Lactobacillus) |
| Fungi | • Multicellular, heterotrophic organisms • Lack chlorophyll and are generally found in colonies • Example – Penicillium, Aspergillus |
| Protozoa | • Unicellular or multicellular microorganisms • Usually found in water • Example – Amoeba and Paramecium |
| Algae | • Unicellular or multicellular autotrophic organisms • Contain chlorophyll pigment and carry out photosynthesis • Example – Chlamydomonas and Spirogyra |
| Viruses | • Ultramicroscopic organisms • Require cells of host organisms to reproduce • Example – Influenza virus, polio virus |
❖Importance of microorganisms
• In food industry
⚬ Lactobacillus bacteria promote the conversion of milk into curd.
⚬ Yeast is used in preparation of breads, pastries, and cakes.
⚬ Yeast is used in preparation of breads, pastries, and cakes.
• In beverage industry
⚬ Yeast acts on sugar and converts into alcohol by the process of fermentation.
⚬ Louis Pasteur discovered fermentation.
⚬ Yeast is used for commercial production of alcohol, wine and vinegar (acetic acid).
⚬ Louis Pasteur discovered fermentation.
⚬ Yeast is used for commercial production of alcohol, wine and vinegar (acetic acid).
• In medicine production
⚬ Medicines produced by certain microorganisms to kill or stop the growth of other disease-causing microorganisms are called antibiotics.
⚬ Antibiotics are obtained from bacteria and fungi.
⚬ Commonly used antibiotics are streptomycin, tetracycline, and erythromycin.
⚬ First antibiotic i.e. penicillin was prepared by Alexander Fleming
⚬ Antibiotics are obtained from bacteria and fungi.
⚬ Commonly used antibiotics are streptomycin, tetracycline, and erythromycin.
⚬ First antibiotic i.e. penicillin was prepared by Alexander Fleming
• In vaccine production
⚬ Protection of the body from the attack of various disease-causing microorganisms through vaccines is known as vaccination.
⚬ Vaccine includes dead or weakened microbes that trigger the production of antibodies in the body.
⚬ These antibodies help in preventing attack from disease-causing microorganisms.
⚬ Vaccination helps in controlling diseases such as cholera, polio, small pox, hepatitis, etc.
⚬ Vaccine for small pox was discovered by Edward Jenner
⚬ Vaccine includes dead or weakened microbes that trigger the production of antibodies in the body.
⚬ These antibodies help in preventing attack from disease-causing microorganisms.
⚬ Vaccination helps in controlling diseases such as cholera, polio, small pox, hepatitis, etc.
⚬ Vaccine for small pox was discovered by Edward Jenner
• In increasing soil fertility
⚬ Blue green algae and Rhizobium bacteria are called biological nitrogen fixers.
⚬ They fix atmospheric free nitrogen to enhance soil fertility.
⚬ They fix atmospheric free nitrogen to enhance soil fertility.
• In cleaning the environment
⚬ Microorganisms (decomposers) help in converting dead waste of plants and animals into simple substances by the process of decomposition.
❖ Harmful microorganisms – Disease-causing microorganisms are called pathogens.
• Diseases in humans caused by microorganisms
⚬ Diseases caused by microorganisms that spread from an infected person to a healthy person through air, water, or food are called communicable diseases.
⚬ The example includes cholera, chicken pox, and tuberculosis
⚬ The organisms that transmit diseases from one place to the other are called carriers.
⚬ Example of carriers:
⚬ The example includes cholera, chicken pox, and tuberculosis
⚬ The organisms that transmit diseases from one place to the other are called carriers.
⚬ Example of carriers:
| Carrier | Disease spread |
| Housefly | Cholera, dysentery and typhoid. |
| Female Anopheles | Malaria |
| Female Aedes | Dengue |
• Examples of communicable diseases
| Disease | Causative agent |
| Tuberculosis | Bacteria |
| Chicken pox | Virus |
| Hepatitis-B | Virus |
| Malaria | Protozoa |
| Cholera | Bacteria |
| Measles | Virus |
| Typhoid | Bacteria |
| Polio | Virus |
• Diseases in animals caused by microorganisms
⚬ Anthrax is caused by bacteria
⚬ Foot and mouth disease in cattle is caused by virus
⚬ Foot and mouth disease in cattle is caused by virus
• Diseases in plants caused by microorganisms
⚬ Citrus canker disease is caused by bacteria
⚬ Rust of wheat is caused by fungi
⚬ Yellow mosaic of Bhindi is caused by virus
⚬ Rust of wheat is caused by fungi
⚬ Yellow mosaic of Bhindi is caused by virus
❖Food preservation
• Microorganisms act on food items and spoil them.
• Process of preventing the spoilage of food items by the action of microbes is called food preservation.
• Process of preventing the spoilage of food items by the action of microbes is called food preservation.
❖Methods of food preservation
• Chemical methods
⚬ The chemical that controls the growth of microorganisms on food are called preservatives. For example, sodium benzoate and sodium meta bisulphate
⚬ Common salt is used as preservative in pickles. It is also used to preserve meat and fish.
⚬ Sugar is used as preservative in jams and jellies.
⚬ Oil and vinegar are used as preservatives in pickles and vegetables.
⚬ Common salt is used as preservative in pickles. It is also used to preserve meat and fish.
⚬ Sugar is used as preservative in jams and jellies.
⚬ Oil and vinegar are used as preservatives in pickles and vegetables.
• Heat and cold treatments
⚬ Boiling the milk helps in killing microorganisms that are present in food.
⚬ Pasteurization is a technique of preserving milk in which it is boiled to about 70°C for 15 to 30 seconds and then suddenly chilled and stored.
⚬ Pasteurization is a technique of preserving milk in which it is boiled to about 70°C for 15 to 30 seconds and then suddenly chilled and stored.
• Storage and packing: Dry fruits and vegetables are sealed in air tight packets to prevent microbe attack.
❖Nitrogen cycle: It involves the circulation of nitrogen through living and non-living components of nature.
• Nitrogen gas comprises 78% of the atmosphere.
• First process of nitrogen cycle is fixation of nitrogen gas into nitrogenous compounds caused by Rhizobium bacteria and lightning.
• Nitrogen compounds in soil are taken up by plants through roots and used up in synthesis of plant proteins. Animals obtain nitrogen by feeding on plants.
• Waste of plants and animals are converted to nitrogenous compounds by the action of bacteria and fungi in the soil.
• Some bacteria convert nitrogenous compounds back to nitrogen to maintain atmospheric levels of nitrogen.
• First process of nitrogen cycle is fixation of nitrogen gas into nitrogenous compounds caused by Rhizobium bacteria and lightning.
• Nitrogen compounds in soil are taken up by plants through roots and used up in synthesis of plant proteins. Animals obtain nitrogen by feeding on plants.
• Waste of plants and animals are converted to nitrogenous compounds by the action of bacteria and fungi in the soil.
• Some bacteria convert nitrogenous compounds back to nitrogen to maintain atmospheric levels of nitrogen.
