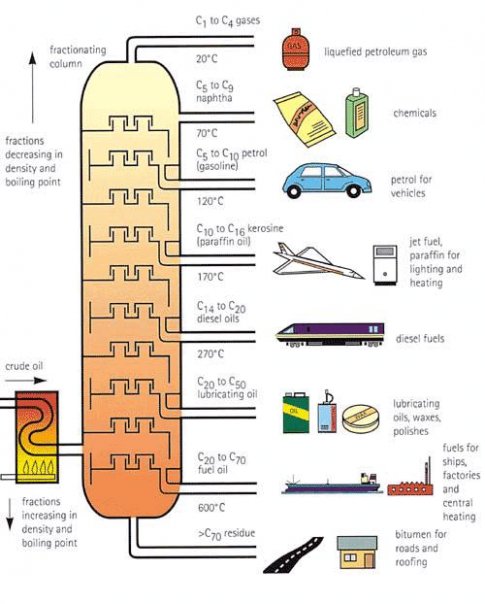
Explain the principle of working of fractional distillation tower with the help of an diagram. Show the products obtained at different levels as a result of fractional distillation of petroleum. Name any 2 products not used as a fuel.
Fractional distillation is similar to distillation where the mixture of liquid is separated in different fraction at different temperature (depend upon boiling point of liquid.)
Fractional distillation tower is long column where mixture is introduced at the bottom of the column and it is heated at high temperature.When heating is on then the vapour of the liquids will form and will evaporate at different rates and a mixture of vapours will produced in the column.
The vapour enters the bottom of the column that is filled with trays or plates. The trays have many holes or bubble caps (like a loosened cap on a soda bottle) in them to allow the vapor to pass through. They increase the contact time between the vapor and the liquids in the column and help to collect liquids at various heights in the column.
There is a temperature difference across the column (hot at the bottom, cool at the top).When a substance in the vapor reaches a height where the temperature of the column is equal to that substance's boiling point, it will condense to form a liquid. (The substance with the lowest boiling point will condense at the highest point in the column; substances with higher boiling points will condense lower in the column).
The trays collect the various liquid fractions.
The collected liquid fractions may pass to condensers, which cool them further, and then go to storage tanks, or they may go to other areas for further chemical processing. The diagram shown below is the fractionating column used to obtian petroleum from crude oil.
The products obtained at different level is shown in diagram. The two products which are not used as fuel is lubricating oil and bitumen.