find bond order and formal charge of SO4-2 and PO4 -3
Draw the resonance structures for SO4 2-
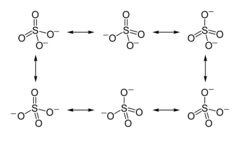
between any two atoms : overall bond order = bond order in each resonance structure / no. of such structures
concentrate on one single S-O bond. There is total 9 bonds on any of S-O with in 6 structures.
so the bond order is = 9/6 = 1.5
formal charge can be calculated as:No. of valence electrons - No.of non bonding electrons - (1/2) of bonding electrons.
For Sulfur : 6-0-12/2= 0
similarly you can calculate any of Oxygen
For PO43- draw the resonance structure
Bond order = 6/5 = 1.2
formal charge on P = 5 - 0-10/2 = 0
similarly you can calculate for any of oxygen.

