In a symbiotic relationship with the soil bacteria known as 'rhizobia', legumes form nodules on their roots (or stems, see figure below) to 'fix' nitrogen into a form usable by plants (and animals). The process of biological nitrogen fixation was discovered by the Dutch microbiologist Martinus Beijerinck. Rhizobia (e.g.,Rhizobium, Mesorhizobium, Sinorhizobium) fix atmospheric nitrogen or dinitrogen, N2, into inorganic nitrogen compounds, such as ammonium, NH4+, which is then incorporated into amino acids, which can be utilized by the plant. Plants cannot fix nitrogen on their own, but need it in one form or another to make amino acids and proteins. Because legumes form nodules with rhizobia, they have high levels of nitrogen available to them. Their abundance of nitrogen is beneficial not only to the legumes themselves, but also to the plants around them. There are other sources of nitrogen in the soil, but are not always provided at the levels required by plants, making the symbiotic relationship between legumes and rhizobia highly beneficial. In return for the fixed nitrogen that they provide, the rhizobia are provided shelter inside of the plant's nodules and some of the carbon substrates and micronutrients that they need to generate energy and key metabolites for the cellular processes that sustain life (Sprent, 2001). Nodulation and nitrogen fixation by rhizobia is not exclusive to legumes; rhizobia form root nodules onParasponisMiq., a genus of five species in the Ulmaceae (see 'Rosales').

bm_27-03.250a.jpg)

The picture on the left shows typical root nodules, these from bur clover (Medicago). The picture on the right shows "stem" nodules onSesbania rostrata- stem nodules are produced from lateral or adventitious roots and are typically found in those few water-tolerant legume groups (Neptunia, Sesbania) that prefer wet or water-logged soils (Goormachtig et al., 2004).
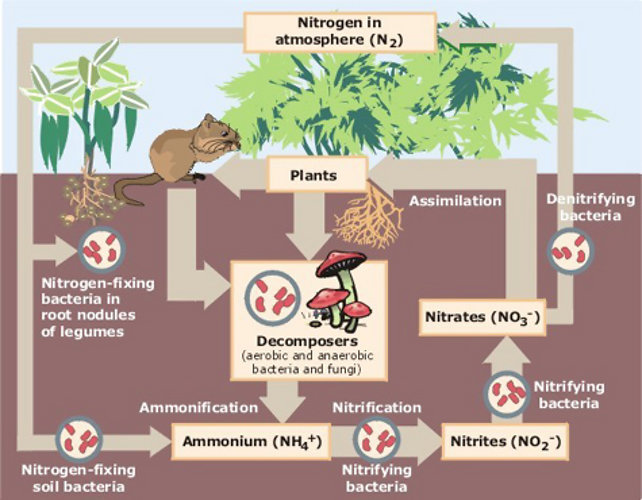
The nitrogen cycle (shown below) describes the series of processes by which the element nitrogen, which makes up about 78% of the Earths atmosphere, cycles between the atmosphere and the biosphere. Plants, bacteria, animals, and manmade and natural phenomena all play a role in the nitrogen cycle. The fixation of nitrogen, in which the gaseous form dinitrogen, N2) is converted into forms usable by living organisms, occurs as a consequence of atmospheric processes such as lightning, but most fixation is carried out by free-living and symbiotic bacteria. Plants and bacteria participate in symbiosis such as the one between legumes and rhizobia or contribute through decomposition and other soil reactions. Bacteria likeRhizobium, or the actinomyceteFrankiawhich nodulates members of the plant families Rosaceae and Betulaceae, utilize atmospheric nitrogen and convert it to an inorganic form (usually ammonium, NH4+) that plants can use. The plants then use the fixed nitrogen to produce vital cellular products such as proteins. The plants are then eaten by animals, which also need nitrogen to make amino acids and proteins. Decomposers acting on plant and animal materials and waste return nitrogen back to the soil. Human-produced fertilizers are another source of nitrogen in the soil along with pollution and volcanic emissions, which release nitrogen into the air in the form of ammonium and nitrate gases. The gases react with the water in the atmosphere and are absorbed by the soil with rain water. Other bacteria in the soil are key components in this cycle converting nitrogen containing compounds to ammonia, NH3, nitrates, NO3-, and nitrites, NO2-. Nitrogen is returned back to the atmosphere by denitrifying bacteria, which convert nitrates to dinitrogen gas.




bm_27-03.250a.jpg)

