what is the simpliest way to know or analyze the rutherford scattering formula? simple equation with corresponding explanation of it.
Thescatteringof alpha particles from nuclei can be modeled from theCoulomb forceand treated as an orbit. Thescatteringprocess can be treated statistically in terms of thecross-section for interactionwith a nucleus which is considered to be a point charge Ze. For a detector at a specific angle with respect to the incident beam, the number of particles per unit area striking the detector is given by the Rutherford formula:
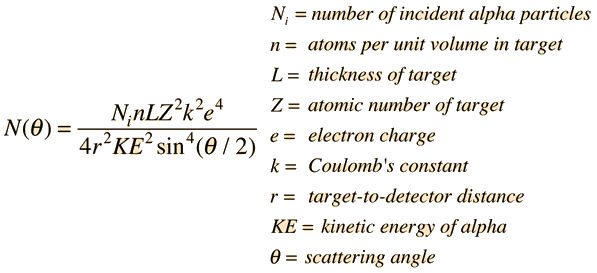
The predicted variation of detected alphas with angle is followed closely by theGeiger-Marsdendata. The above form includes the cross-section for scattering for a given nucleus and the nature of the scattering film to get thescattered fraction. Another common form for the Rutherford equation is just the differentialcross sectionfor scattering from a given nucleus.
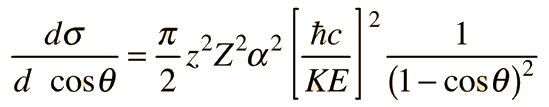
For this equation, some of the constants have been combined to express the cross section in terms of thefine-structure constant,a.
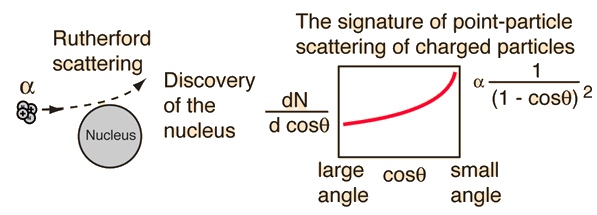
This form of the scattering formula serves as a signature for scattering off a point target in which no structure is evident. The point ofdeparture from Rutherford scatteringin the case of the nucleus was the basis for the earliest evaluations of the nuclear radius.
Thedeparture from the point-particle formof scattering has been an indicator of nuclear structure and then at higher energies, the structure of the proton.

