Q14. Predict the nature of following salts by hydrolysing them, and give chemical equations: (3)
a) Sodium chloride.
b) Magnesium sulphate.
c) Potassium carbonate.
Dear Student
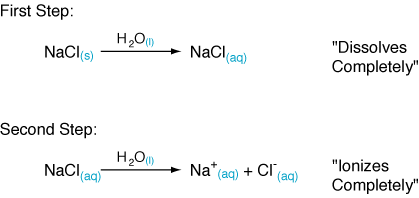
Sodium chloride, formed from the neutralization of HCl by NaOH. A solution of NaCl in water has no acidic or basic properties, since neither ion is capable of hydrolyzing. NaCl ionizes completely when dissolved in water. It's helpful to think of this process as two steps:
When dissolved in water, magnesium sulfate ionizes (separates) into magnesium (Mg2+) ions and sulfate (SO42-) ions. Solutions of magnesium sulfate have a neutral pH.
MgSO4(+H2O) -> (Mg++)(aq) + (SO4- -)(aq)
Sodium chloride, formed from the neutralization of HCl by NaOH. A solution of NaCl in water has no acidic or basic properties, since neither ion is capable of hydrolyzing. NaCl ionizes completely when dissolved in water. It's helpful to think of this process as two steps:

When dissolved in water, magnesium sulfate ionizes (separates) into magnesium (Mg2+) ions and sulfate (SO42-) ions. Solutions of magnesium sulfate have a neutral pH.
MgSO4(+H2O) -> (Mg++)(aq) + (SO4- -)(aq)
K2CO3 →2K++ CO3-²
K+ + OH- (ion from water)→ KOH Strong base
2H+ + CO3 → H2CO3 ( this acid formed is a weak acid and is less ionized)
while KOH is strong base and is highly ionized.
therefore solution becomes strongly basic.
Regards

