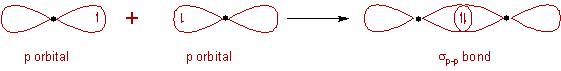state potuelates of valence bond theory
rn
The Valence Bond Theory was introduced by Heitler and London (1927); developed further by Pauling and others. This theory is basically concerned with the energetics of covalent bond formation about which lewis and VSEPR models are silent.
1) The atomic orbitals of two atoms overlap with each other to form a bond. Greater is the overlapping, stronger is the bond formed. The potential energy of the system gets lowered as the two atoms come close to each other.
3) At the equilibrium inter nuclear distance (bond distance) the energy touches a minimum. Any attempt to bring the nuclei still closer results in a sudden increase in energy and consequently destabilisation of molecule.
4) Because of orbital overlap electron density increases between the nuclei, which brings them together.
5) The direction of covalent bond is along the region of overlap of atomic orbitals i.e. covalent bond is directional.
6) There are two types of covalent bond, depending on the nature of overlap - σ bond and π bond .
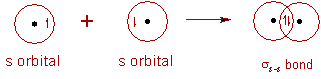
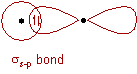
7) σ bond is formed by the head-to-head overlap of orbitals along the inter-nuclear axis. These are of three types s-s, s-p and p-p
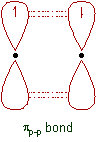
8) π bond formed by the sideways overlap of orbitals. P, d and f orbitals form π bonds by sideways overlap.
9) It also takes into account the concept of Hybridisation. It states that atomic orbitals of slightly different energies combine to form a new set of equivalent orbitals (having the same energy and shape) known as hybrid orbitals.
These hybrid orbitals are more stable than atomic orbitals, and participate in bond formation.
The VB theory and concept of Hybridisation can also be applied to co-ordination compounds. The basic principles are
1) The metal atom or ion under the influence of ligands can use its (n−1)d, ns, np or ns, np, nd orbitals for hybridisation, to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, square planar, and so on.
2) These hybridised orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.