what is a acidic buffer solution and how can it be prepared?
Buffer Solution is a solution which resists change in hydrogen ion concentration (and, thereby pH) on dilution, or with the addition of a small amount of an acid or a base
An acidic buffer solution is simply one which has a pH less than 7. Acidic buffer solutions are commonly made from a weak acid and one of its salts. The common example would be a mixture of ethanoic acid and sodium ethanoate in solution. In this case, if the solution contained equal molar concentrations of both the acid and the salt, it would have a pH of 4.76.The pH of the buffer can change by changing the ratio of acid to salt, or by choosing a different acid and one of its salts.
Ethanoic acid is a weak acid, and the position of this equilibrium will be well to the left:

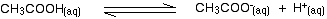
Adding sodium ethanoate to this adds lots of extra ethanoate ions. According to Le Chatelier's Principle, that will shift the position of the equilibrium even further to the left.
The solution will therefore contain these important things:
- lots of un-ionised ethanoic acid;
- lots of ethanoate ions from the sodium ethanoate;
- enough hydrogen ions to make the solution acidic.

