what is real gas equation ie vander walls equation?
Real gases are non-hypothetical gases whose molecules occupy space and have interactions; consequently, they adhere to gas laws.
There are two corrective factors in van der Waals equation. The first, , alters the pressure in the ideal gas equation. It accounts for the intermolecular attractive forces between gas molecules. The magnitude of a is indicative of the strength of the intermolecular attractive force.
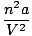 )(V - nb) = nRT
)(V - nb) = nRT
There are two corrective factors in van der Waals equation. The first, , alters the pressure in the ideal gas equation. It accounts for the intermolecular attractive forces between gas molecules. The magnitude of a is indicative of the strength of the intermolecular attractive force.
The van der Waals equation corrects for the volume of, and attractive forces between, gas molecules:
(P +
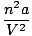 )(V - nb) = nRT
)(V - nb) = nRT


