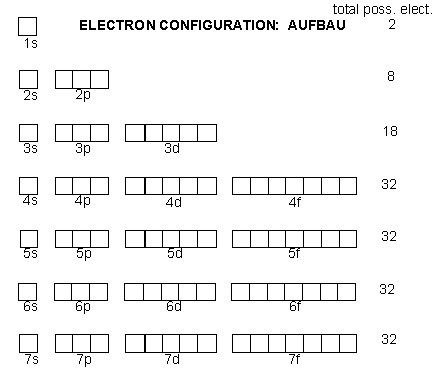
Why are there 18 elements in the fourth block of the modern periodic table?
The 1st energy level (K shell) has only 1 orbital which can accommodate 2 electrons hence 2 elements in 1st period of periodic table.
2nd energy level has 4 orbitals in 2 sub-shell hence can accommodate 8 electrons and therefore 8 elements in 2nd period.
3rd energy level has 9 orbitals in 3 sub-shell hence can accommodate 18 electrons. But only 4 orbitals get filled (due to some energy level rules which you will study in higher classes).Hence 8 elements in 3rd period.
Similarly 9 orbitals get filled in 4th energy level hence 18 elements are present in 4th period of periodic table.The diagram shown below the no. of orbitals in each energy level and no. of possible elctrons.(here 1s,2s and 2p are subshell and orbitals as square box.)