Why partial charges are always less than the unit electronic charge (1.6 x 10^-19).explain this in detail with a suitable example. it is my humble request please don't send a link
Dear Student,
Firstly I would like to explain the meaning of partial charge.
Partial charge means that the element or atom which posses a partial charge do not have complete charge charge. It will be more clear with the given below example:
For example :
H2O
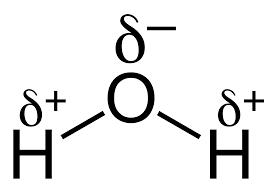
In case of water molecule Hydrogen atom and oxygen both have charges i.e. oxygen has partial negative and hydrogen has partial positive charge.
It means the charge on the oxygen is not complete negative. Partial negative charge here refer to the fact that being a highly electronegative it withdraws the negative charge (electron density) of shared pair of electron toward itself which results in partial negative charge on it,
And its value cannot be equal to the charge and it will the less than the unity charge.
Reason: Although electron density is near the vicinity of oxygen but it is also shared by the hydrogen atom.
Hope this will help you and if you still faces any doubt in the topic do let me Know.
Regards
Firstly I would like to explain the meaning of partial charge.
Partial charge means that the element or atom which posses a partial charge do not have complete charge charge. It will be more clear with the given below example:
For example :
H2O
In case of water molecule Hydrogen atom and oxygen both have charges i.e. oxygen has partial negative and hydrogen has partial positive charge.
It means the charge on the oxygen is not complete negative. Partial negative charge here refer to the fact that being a highly electronegative it withdraws the negative charge (electron density) of shared pair of electron toward itself which results in partial negative charge on it,
And its value cannot be equal to the charge and it will the less than the unity charge.
Reason: Although electron density is near the vicinity of oxygen but it is also shared by the hydrogen atom.
Hope this will help you and if you still faces any doubt in the topic do let me Know.
Regards

