how to draw lewis sructures of H3P3O9,H4P4O12,H4P2O7?
The structure of the given molecules will be drawn following the given rules.
There are 6 steps to write lewis structure:
STEP 1. For a given chemical formula, count the number of valence electrons for each atom . Add them up. If the system is charged, add one for each negative charge on the system or subtract one for each positive charge on the system.
STEP 2. Determine the arrangement of the atoms.Assume that the least electronegative atom is central atom.
STEP 3. Place single bonds between the atoms as determined in step 2.
STEP 4. Multiply the number of single bonds in step 3 by two and subtract that number from the number determined in step 1. This gives the number of available electrons.
STEP 5. Arrange the available electrons in pairs around the terminal (outside) atoms, except hydrogen, to fulfil the octet rule. Arrange any remaining electrons around the central atom(s) to fulfil the octet rule. If all of the atoms except hydrogen have an octet of electrons then you are finished.
STEP 6. If necessary to complete the central atom octet move electron pairs from the terminal atoms to form multiple bonds with the central atoms.
All the given molecule are the type of phosphoric acid. lewis structure of phosphoric acid is shown below following above rules.
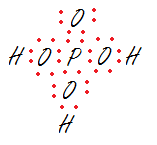

H2P4O7 is formed by addition of two phosphoric acid molecule and removal of water from them.In lewis structure will be similar to phosphoric acid.
H3P3O9 and H4P4O12 are cylic phosphoric acids form by removal of 2 water molecules from each phosphoric acid molecule. In lewis structure each of the O will carry the 2 lone pairs of electrons. The basic structure will be same.

There are 6 steps to write lewis structure:
STEP 1. For a given chemical formula, count the number of valence electrons for each atom . Add them up. If the system is charged, add one for each negative charge on the system or subtract one for each positive charge on the system.
STEP 2. Determine the arrangement of the atoms.Assume that the least electronegative atom is central atom.
STEP 3. Place single bonds between the atoms as determined in step 2.
STEP 4. Multiply the number of single bonds in step 3 by two and subtract that number from the number determined in step 1. This gives the number of available electrons.
STEP 5. Arrange the available electrons in pairs around the terminal (outside) atoms, except hydrogen, to fulfil the octet rule. Arrange any remaining electrons around the central atom(s) to fulfil the octet rule. If all of the atoms except hydrogen have an octet of electrons then you are finished.
STEP 6. If necessary to complete the central atom octet move electron pairs from the terminal atoms to form multiple bonds with the central atoms.
All the given molecule are the type of phosphoric acid. lewis structure of phosphoric acid is shown below following above rules.
H2P4O7 is formed by addition of two phosphoric acid molecule and removal of water from them.In lewis structure will be similar to phosphoric acid.

H3P3O9 and H4P4O12 are cylic phosphoric acids form by removal of 2 water molecules from each phosphoric acid molecule. In lewis structure each of the O will carry the 2 lone pairs of electrons. The basic structure will be same.


