Polar molecules result from the unequal sharing of electrons, that is the side that has more of its share of the electrons will be more negative, and therefore polar. Ionic bonds are formed due to the attraction between an atom that has lost one or more electron (known as a cation) and an atom that has gained one or more electrons (known as an anion). Usually, the cation is a metal atom and the anion is a nonmetal atom. All ionic bonds are highly polar. The term "ionic bond" is given to a bond in which the ionic character is greater than the covalent character - that is, a bond in which a large electronegativity difference exists between the two atoms, causing the bond to be more polar (ionic) than other forms of covalent bonding where electrons are shared more equally. Bonds with partially ionic and partially covalent character are called polar covalent bonds.
Only the alkali and alkaline earth metals along with the halogens, oxygen and sometimes nitrogen form bonds that are classically ionic.
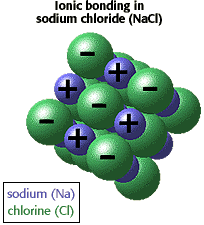
Bonding in ionic compounds : Ionic compounds in the solid state form lattice structures. What gives NaCl its properties that we normally associate with ionic compounds is the network structure of the solid where sodium and chlorine atoms alternate in three dimensions. This type of bonding is much stronger than dipole-dipole interactions in polar molecules.

Below is weaker dipole-dipole interactions between polar molecules.





