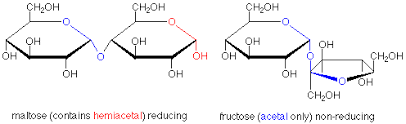
Difference between Reducing Sugar and Non-reducing sugar is in its structure. Reducing sugars have a aldehyde group or carbonyl functional group, which can be reduced to -OH group by chemical reaction. Some sugars like Fructose get re-arranged in specific conditions like alkaline pH, and form a compound that is reducible. Most common reducing agents to decide this nature are Tollen's reagent, Fehling's reagent, Benedict's reagent, etc. Maltose has an aldehyde group in them, and that is why it is a reducing sugar.
Non-reducing sugars do not reduce the above because of absence of an aldehyde group. (E.g. sucrose)
Fructose does not have an aldehyde group, yet it is reducing, because it gets rearranged to the reducing glucose in basic solution.
Reducing sugars are monosaccarides and disaccarides. If my memory serves me right they discolour benedicts solutions because the above sugars have a Hydroxyl group. This group reacts with the copper in the benedicts solution changing it's colour.
Non reducing sugars are polysaccarides - they do not have the hydroxyl group and therefore have to be refluxed in acid to obtain a similar reactive group.