19 in a compound

Dear Student,
Please find below the solution of your asked query:
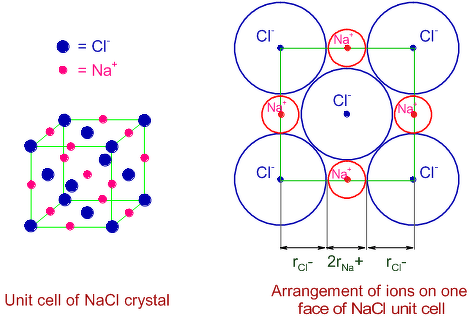
In case of NaCl , two Cl⁻ ions present at corner of NaCl lattice and one Na⁺ ion present at edge of NaCl lattice. So, the edge length is the sum of two radius of Cl⁻ ion and two radius of Na⁺ ion as you can see in attachment.
∴ 2 × radius of Cl⁻ ion + 2 × radius of Na⁺ ion = edge length
Given, distance between Na⁺ and Cl⁻ = x pm
means , (radius of Na⁺ + radius of Cl⁻) = x pm
∴ 2(radius of Na⁺ + radius of Cl⁻) = edge length
⇒ 2x pm = edge length
Hope this information clears your doubts about the topic.
Keep asking!!
Regards
Please find below the solution of your asked query:
In case of NaCl , two Cl⁻ ions present at corner of NaCl lattice and one Na⁺ ion present at edge of NaCl lattice. So, the edge length is the sum of two radius of Cl⁻ ion and two radius of Na⁺ ion as you can see in attachment.
∴ 2 × radius of Cl⁻ ion + 2 × radius of Na⁺ ion = edge length
Given, distance between Na⁺ and Cl⁻ = x pm
means , (radius of Na⁺ + radius of Cl⁻) = x pm
∴ 2(radius of Na⁺ + radius of Cl⁻) = edge length
⇒ 2x pm = edge length

Hope this information clears your doubts about the topic.
Keep asking!!
Regards

