how can a square planar complex of MABCD type show trans isomerism (M = metal A, B, C, D = LIGANDS)?
Given below is the example of square planar complex of MABCD type.
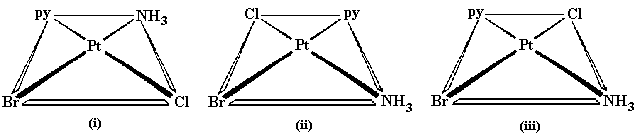
Here Pt is metal and ligands are Br, Py, NH3 and Cl. Keeping the position of Br constant in i) it is trans with NH3, in ii) it is trans with py , in iii) it is trans with Cl. Hence 3 isomer can be written.

