how to determine which compound reacts with NaOH (eg.carboxylic acid,phenol) also same for NaHCO3
Dear Student,
The compounds which can react with NaOH and NaHCO3 can be determined by the acidity of the compounds.
Most acids react with NaOH as it is a strong base but most acids do not completely neutralize NaHCO3 because it is weak in nature.
The lower the pKa, the stronger the acid. Similarly, the lower the pKb, the stronger the base.
For the given example of carboxylic acid and phenol:
Carboxylic acid is a stronger acid than phenol because the negative charge in the conjugate base is more dispersed than in conjugate base of phenol, due to the presence of two O atoms. Also, in the resonance structures, in carboxylic acids, the negative charge is present on the O atoms whereas in phenol, the negative charge is present on the electropositive carbon as well, thus decreasing the stability.
NaOH reacts with both carboxylic acid and phenol because it is a strong base.
But, NaHCO3 reacts with only carboxylic acid and not phenol because both phenol and bicarbonate are weak in nature.
Reactions:
With NaOH:
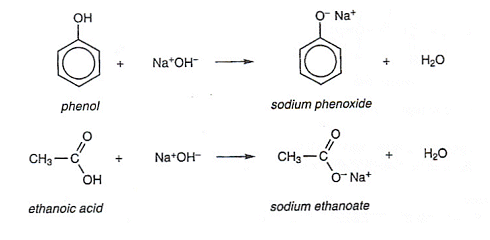
With NaHCO3:

Phenol + NaHCO3 No Reaction
Regards
The compounds which can react with NaOH and NaHCO3 can be determined by the acidity of the compounds.
Most acids react with NaOH as it is a strong base but most acids do not completely neutralize NaHCO3 because it is weak in nature.
The lower the pKa, the stronger the acid. Similarly, the lower the pKb, the stronger the base.
For the given example of carboxylic acid and phenol:
Carboxylic acid is a stronger acid than phenol because the negative charge in the conjugate base is more dispersed than in conjugate base of phenol, due to the presence of two O atoms. Also, in the resonance structures, in carboxylic acids, the negative charge is present on the O atoms whereas in phenol, the negative charge is present on the electropositive carbon as well, thus decreasing the stability.
NaOH reacts with both carboxylic acid and phenol because it is a strong base.
But, NaHCO3 reacts with only carboxylic acid and not phenol because both phenol and bicarbonate are weak in nature.
Reactions:
With NaOH:

With NaHCO3:

Phenol + NaHCO3 No Reaction
Regards

